Core glycosylation of collagen is initiated by two beta(1-O)galactosyltransferases
- PMID: 19075007
- PMCID: PMC2643808
- DOI: 10.1128/MCB.02085-07
Core glycosylation of collagen is initiated by two beta(1-O)galactosyltransferases
Abstract
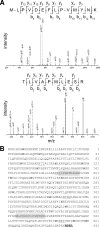
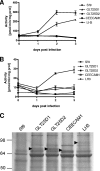
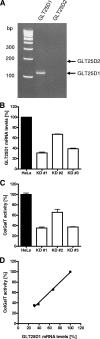
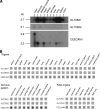
Collagen is a trimer of three left-handed alpha chains representing repeats of the motif Gly-X-Y, where (hydroxy)proline and (hydroxy)lysine residues are often found at positions X and Y. Selected hydroxylysines are further modified by the addition of galactose and glucose-galactose units. Collagen glycosylation takes place in the endoplasmic reticulum before triple-helix formation and is mediated by beta(1-O)galactosyl- and alpha(1-2)glucosyltransferase enzymes. We have identified two collagen galactosyltransferases using affinity chromatography and tandem mass spectrometry protein sequencing. The two collagen beta(1-O)galactosyltransferases corresponded to the GLT25D1 and GLT25D2 proteins. Recombinant GLT25D1 and GLT25D2 enzymes showed a strong galactosyltransferase activity toward various types of collagen and toward the serum mannose-binding lectin MBL, which contains a collagen domain. Amino acid analysis of the products of GLT25D1 and GLT25D2 reactions confirmed the transfer of galactose to hydroxylysine residues. The GLT25D1 gene is constitutively expressed in human tissues, whereas the GLT25D2 gene is expressed only at low levels in the nervous system. The GLT25D1 and GLT25D2 enzymes are similar to CEECAM1, to which we could not attribute any collagen galactosyltransferase activity. The GLT25D1 and GLT25D2 genes now allow addressing of the biological significance of collagen glycosylation and the importance of this posttranslational modification in the etiology of connective tissue disorders.
Figures




Similar articles
-
Identification of domains and amino acids essential to the collagen galactosyltransferase activity of GLT25D1.PLoS One. 2011;6(12):e29390. doi: 10.1371/journal.pone.0029390. Epub 2011 Dec 21. PLoS One. 2011. PMID: 22216269 Free PMC article.
-
Collagen Accumulation in Osteosarcoma Cells lacking GLT25D1 Collagen Galactosyltransferase.J Biol Chem. 2016 Aug 26;291(35):18514-24. doi: 10.1074/jbc.M116.723379. Epub 2016 Jul 11. J Biol Chem. 2016. PMID: 27402836 Free PMC article.
-
The human collagen beta(1-O)galactosyltransferase, GLT25D1, is a soluble endoplasmic reticulum localized protein.BMC Cell Biol. 2010 May 14;11:33. doi: 10.1186/1471-2121-11-33. BMC Cell Biol. 2010. PMID: 20470363 Free PMC article.
-
Identification of Regulatory Molecular "Hot Spots" for LH/PLOD Collagen Glycosyltransferase Activity.Int J Mol Sci. 2023 Jul 7;24(13):11213. doi: 10.3390/ijms241311213. Int J Mol Sci. 2023. PMID: 37446392 Free PMC article. Review.
-
Sequence-function relationships of prokaryotic and eukaryotic galactosyltransferases.J Biochem. 1998 Jun;123(6):1000-9. doi: 10.1093/oxfordjournals.jbchem.a022035. J Biochem. 1998. PMID: 9603985 Review.
Cited by
-
The activities of lysyl hydroxylase 3 (LH3) regulate the amount and oligomerization status of adiponectin.PLoS One. 2012;7(11):e50045. doi: 10.1371/journal.pone.0050045. Epub 2012 Nov 29. PLoS One. 2012. PMID: 23209641 Free PMC article.
-
Effects of fish collagen peptides on collagen post-translational modifications and mineralization in an osteoblastic cell culture system.Dent Mater J. 2013;32(1):88-95. doi: 10.4012/dmj.2012-220. Dent Mater J. 2013. PMID: 23370875 Free PMC article.
-
Rab GTPase mediated procollagen trafficking in ascorbic acid stimulated osteoblasts.PLoS One. 2012;7(9):e46265. doi: 10.1371/journal.pone.0046265. Epub 2012 Sep 26. PLoS One. 2012. PMID: 23050002 Free PMC article.
-
Comprehensive Mapping and Dynamics of Site-Specific Prolyl-Hydroxylation, Lysyl-Hydroxylation and Lysyl O-Glycosylation of Collagens Deposited in ECM During Zebrafish Heart Regeneration.Front Mol Biosci. 2022 Jun 16;9:892763. doi: 10.3389/fmolb.2022.892763. eCollection 2022. Front Mol Biosci. 2022. PMID: 35782869 Free PMC article.
-
The fibrotic tumor stroma.J Clin Invest. 2018 Jan 2;128(1):16-25. doi: 10.1172/JCI93554. Epub 2018 Jan 2. J Clin Invest. 2018. PMID: 29293090 Free PMC article. Review.
References
-
- Bank, R. A., B. Beekman, R. Tenni, and J. M. TeKoppele. 1997. Pre-column derivatisation method for the measurement of glycosylated hydroxylysines of collagenous proteins. J. Chromatogr. B Biomed. Sci. Appl. 703267-272. - PubMed
-
- Bennett, E. P., H. Hassan, U. Mandel, E. Mirgorodskaya, P. Roepstorff, J. Burchell, J. Taylor-Papadimitriou, M. A. Hollingsworth, G. Merkx, A. G. van Kessel, H. Eiberg, R. Steffensen, and H. Clausen. 1998. Cloning of a human UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase that complements other GalNAc-transferases in complete O-glycosylation of the MUC1 tandem repeat. J. Biol. Chem. 27330472-30481. - PubMed
-
- Bon, S., A. Ayon, J. Leroy, and J. Massoulie. 2003. Trimerization domain of the collagen tail of acetylcholinesterase. Neurochem. Res. 28523-535. - PubMed
-
- Cabral, W. A., W. Chang, A. M. Barnes, M. Weis, M. A. Scott, S. Leikin, E. Makareeva, N. V. Kuznetsova, K. N. Rosenbaum, C. J. Tifft, D. I. Bulas, C. Kozma, P. A. Smith, D. R. Eyre, and J. C. Marini. 2007. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat. Genet. 39359-365. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
