m.3243A>G mutation in mitochondrial DNA leads to decreased insulin sensitivity in skeletal muscle and to progressive beta-cell dysfunction
- PMID: 19073775
- PMCID: PMC2646052
- DOI: 10.2337/db08-0981
m.3243A>G mutation in mitochondrial DNA leads to decreased insulin sensitivity in skeletal muscle and to progressive beta-cell dysfunction
Abstract
Objective: To study insulin sensitivity and perfusion in skeletal muscle together with the beta-cell function in subjects with the m.3243A>G mutation in mitochondrial DNA, the most common cause of mitochondrial diabetes.
Research design and methods: We measured skeletal muscle glucose uptake and perfusion using positron emission tomography and 2-[18F]fluoro-2-deoxyglucose and [15O]H2O during euglycemic hyperinsulinemia in 15 patients with m.3243A>G. These patients included five subjects with no diabetes as defined by the oral glucose tolerance test (OGTT) (group 1), three with GHb <6.1% and newly found diabetes by OGTT (group 2), and seven with a previously diagnosed diabetes (group 3). Control subjects consisted of 13 healthy individuals who were similar to the carriers of m.3243A>G with respect to age and physical activity. Beta-cell function was assessed using the OGTT and subsequent mathematical modeling.
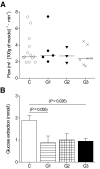
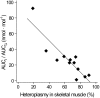
Results: Skeletal muscle glucose uptake was significantly lower in groups 1, 2, and 3 than in the control subjects. The glucose sensitivity of beta-cells in group 1 patients was similar to that of the control subjects, whereas in group 2 and 3 patients, the glucose sensitivity was significantly lower. The insulin secretion parameters correlated strongly with the proportion of m.3243A>G mutation in muscle.
Conclusions: Our findings show that subjects with m.3243A>G are insulin resistant in skeletal muscle even when beta-cell function is not markedly impaired or glucose control compromised. We suggest that both the skeletal muscle insulin sensitivity and the beta-cell function are affected before the onset of the mitochondrial diabetes caused by the m.3243A>G mutation.
Figures

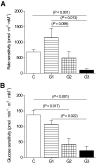
 , group 1;
, group 1;  , group 2; and ▪, group 3. The apparently high rate sensitivity in group 1 is partly due to the higher insulin sensitivity in the control subjects, because rate sensitivity tends to be inversely correlated with insulin sensitivity (see Table 2 for respective disposition index). B: Glucose sensitivity is the insulin dose-response function to absolute glucose level during OGTT.
, group 2; and ▪, group 3. The apparently high rate sensitivity in group 1 is partly due to the higher insulin sensitivity in the control subjects, because rate sensitivity tends to be inversely correlated with insulin sensitivity (see Table 2 for respective disposition index). B: Glucose sensitivity is the insulin dose-response function to absolute glucose level during OGTT.
Similar articles
-
Mitochondrial diabetes is associated with insulin resistance in subcutaneous adipose tissue but not with increased liver fat content.J Inherit Metab Dis. 2011 Dec;34(6):1205-12. doi: 10.1007/s10545-011-9338-0. Epub 2011 May 10. J Inherit Metab Dis. 2011. PMID: 21556834 Clinical Trial.
-
Myocardial glucose uptake in patients with the m.3243A > G mutation in mitochondrial DNA.J Inherit Metab Dis. 2016 Jan;39(1):67-74. doi: 10.1007/s10545-015-9865-1. Epub 2015 Jun 26. J Inherit Metab Dis. 2016. PMID: 26112752
-
Glucose metabolism derangements in adults with the MELAS m.3243A>G mutation.Mitochondrion. 2014 Sep;18:63-9. doi: 10.1016/j.mito.2014.07.008. Epub 2014 Jul 30. Mitochondrion. 2014. PMID: 25086207 Free PMC article.
-
New insights in the molecular pathogenesis of the maternally inherited diabetes and deafness syndrome.Endocrinol Metab Clin North Am. 2006 Jun;35(2):385-96, x-xi. doi: 10.1016/j.ecl.2006.02.014. Endocrinol Metab Clin North Am. 2006. PMID: 16632100 Review.
-
[Mitochondrial disease caused by the m.3243A>G mutation].Tidsskr Nor Laegeforen. 2022 Jun 27;142(10). doi: 10.4045/tidsskr.21.0729. Print 2022 Jun 28. Tidsskr Nor Laegeforen. 2022. PMID: 35763848 Review. Norwegian.
Cited by
-
Troponin T3 associates with DNA consensus sequence that overlaps with p53 binding motifs.Exp Gerontol. 2018 Jul 15;108:35-40. doi: 10.1016/j.exger.2018.03.012. Epub 2018 Mar 27. Exp Gerontol. 2018. PMID: 29596868 Free PMC article.
-
Not quite type 1 or type 2, what now? Review of monogenic, mitochondrial, and syndromic diabetes.Rev Endocr Metab Disord. 2018 Mar;19(1):35-52. doi: 10.1007/s11154-018-9446-3. Rev Endocr Metab Disord. 2018. PMID: 29777474 Review.
-
Endocrine Manifestations and New Developments in Mitochondrial Disease.Endocr Rev. 2022 May 12;43(3):583-609. doi: 10.1210/endrev/bnab036. Endocr Rev. 2022. PMID: 35552684 Free PMC article.
-
Penetrance and expressivity of mitochondrial variants in a large clinically unselected population.Hum Mol Genet. 2024 Feb 18;33(5):465-474. doi: 10.1093/hmg/ddad194. Hum Mol Genet. 2024. PMID: 37988592 Free PMC article.
-
Hormonal dysfunction in adult patients affected with inherited metabolic disorders.J Mother Child. 2020 Nov 10;24(2):21-31. doi: 10.34763/jmotherandchild.20202402si.2018.000005. J Mother Child. 2020. PMID: 33179602 Free PMC article. Review.
References
-
- Iozzo P, Hällsten K, Oikonen V, Virtanen KA, Kemppainen J, Solin O, Ferrannini E, Knuuti J, Nuutila P: Insulin-mediated hepatic glucose uptake is impaired in type 2 diabetes: evidence for a relationship with glycemic control. J Clin Endocrinol Metab 88: 2055–2060, 2003 - PubMed
-
- Virtanen KA, Lönnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, Tolvanen T, Knuuti J, Rönnemaa T, Huupponen R, Nuutila P: Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab 87: 3902–3910, 2002 - PubMed
-
- Beck-Nielsen H, Vaag A, Poulsen P, Gaster M: Metabolic and genetic influence on glucose metabolism in type 2 diabetic subjects: experiences from relatives and twin studies. Best Pract Res Clin Endocrinol Metab 17: 445–467, 2003 - PubMed
-
- Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG: Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322: 223–228, 1990 - PubMed
-
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ: Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 100: 8466–8471, 2003 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

