Enhanced sensitivity to group II mGlu receptor activation at corticostriatal synapses in mice lacking the familial parkinsonism-linked genes PINK1 or Parkin
- PMID: 19071114
- PMCID: PMC2796563
- DOI: 10.1016/j.expneurol.2008.11.001
Enhanced sensitivity to group II mGlu receptor activation at corticostriatal synapses in mice lacking the familial parkinsonism-linked genes PINK1 or Parkin
Abstract
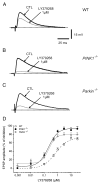
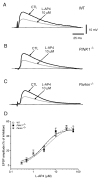
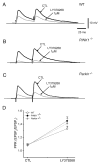
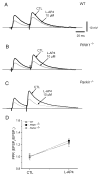
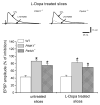
An altered glutamatergic input at corticostriatal synapses has been shown in experimental models of Parkinson's disease (PD). In the present work, we analyzed the membrane and synaptic responses of striatal neurons to metabotropic glutamate (mGlu) receptor activation in two different mouse models of inherited PD, linked to mutations in PINK1 or Parkin genes. Both in PINK1 and Parkin knockout ((-/-)) mice, activation of group I mGlu receptors by 3,5-DHPG caused a membrane depolarization coupled to an increase in firing frequency in striatal cholinergic interneurons that was comparable to the response observed in the respective wild-type (WT) interneurons. The sensitivity to group II and III mGlu receptors was tested on cortically-evoked excitatory postsynaptic potentials (EPSPs) recorded from medium spiny neurons (MSNs). Both LY379268 and L-AP4, agonists for group II and III, respectively, had no effect on intrinsic membrane properties, but dose-dependently reduced the amplitude of corticostriatal EPSPs. However, both in PINK1(-/-) and Parkin(-/-) mice, LY379268, but not L-AP4, exhibited a greater potency as compared to WT in depressing EPSP amplitude. Accordingly, the dose-response curve for the response to LY379268 in both knockout mice was shifted leftward. Moreover, consistent with a presynaptic site of action, both LY379268 and L-AP4 increased the paired-pulse ratio either in PINK1(-/-) and Parkin(-/-) or in WT mice. Acute pretreatment with L-dopa did not rescue the enhanced sensitivity to LY379268. Together, these results suggest that the selective increase in sensitivity of striatal group II mGlu receptors represents an adaptive change in mice in which an altered dopamine metabolism has been documented.
Figures


Similar articles
-
Dopamine-dependent CB1 receptor dysfunction at corticostriatal synapses in homozygous PINK1 knockout mice.Neuropharmacology. 2016 Feb;101:460-70. doi: 10.1016/j.neuropharm.2015.10.021. Epub 2015 Oct 20. Neuropharmacology. 2016. PMID: 26498506
-
Activation of group II metabotropic glutamate receptors induces long-term depression of excitatory synaptic transmission in the substantia nigra pars reticulata.Neurosci Lett. 2011 Oct 24;504(2):102-106. doi: 10.1016/j.neulet.2011.09.007. Epub 2011 Sep 16. Neurosci Lett. 2011. PMID: 21945652 Free PMC article.
-
Striatal metabotropic glutamate receptor function following experimental parkinsonism and chronic levodopa treatment.Brain. 2002 Dec;125(Pt 12):2635-45. doi: 10.1093/brain/awf269. Brain. 2002. PMID: 12429591
-
Activation of group III metabotropic glutamate receptors depresses glutamatergic transmission at corticostriatal synapse.Neuropharmacology. 1997 Jun;36(6):845-51. doi: 10.1016/s0028-3908(96)00177-3. Neuropharmacology. 1997. PMID: 9225312
-
Metabotropic glutamate receptors and cell-type-specific vulnerability in the striatum: implication for ischemia and Huntington's disease.Exp Neurol. 1999 Jul;158(1):97-108. doi: 10.1006/exnr.1999.7092. Exp Neurol. 1999. PMID: 10448421
Cited by
-
Parkinson's disease mouse models in translational research.Mamm Genome. 2011 Aug;22(7-8):401-19. doi: 10.1007/s00335-011-9330-x. Epub 2011 May 11. Mamm Genome. 2011. PMID: 21559878 Free PMC article. Review.
-
Metabotropic glutamate receptor 2 inhibits thalamically-driven glutamate and dopamine release in the dorsal striatum.Neuropharmacology. 2017 May 1;117:114-123. doi: 10.1016/j.neuropharm.2017.01.038. Epub 2017 Jan 31. Neuropharmacology. 2017. PMID: 28159646 Free PMC article.
-
Parkinson's disease: experimental models and reality.Acta Neuropathol. 2018 Jan;135(1):13-32. doi: 10.1007/s00401-017-1788-5. Epub 2017 Nov 18. Acta Neuropathol. 2018. PMID: 29151169 Free PMC article. Review.
-
Nigral overexpression of alpha-synuclein in the absence of parkin enhances alpha-synuclein phosphorylation but does not modulate dopaminergic neurodegeneration.Mol Neurodegener. 2015 Jun 23;10:23. doi: 10.1186/s13024-015-0017-8. Mol Neurodegener. 2015. PMID: 26099628 Free PMC article.
-
Genes Implicated in Familial Parkinson's Disease Provide a Dual Picture of Nigral Dopaminergic Neurodegeneration with Mitochondria Taking Center Stage.Int J Mol Sci. 2021 Apr 28;22(9):4643. doi: 10.3390/ijms22094643. Int J Mol Sci. 2021. PMID: 33924963 Free PMC article. Review.
References
-
- Albanese A, Valente EM, Romito LM, Bellacchio E, Elia AE, Dallapiccola B. The PINK1 phenotype can be indistinguishable from idiopathic Parkinson disease. Neurology. 2005;64 (11):1958–1960. - PubMed
-
- Battaglia G, Busceti CL, Pontarelli F, Biagioni F, Fornai F, Paparelli A, Bruno V, Ruggieri S, Nicoletti F. Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology. 2003;45 (2):155–166. - PubMed
-
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. - PubMed
-
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

