Two arginine-glutamate ionic locks near the extracellular surface of FFAR1 gate receptor activation
- PMID: 19068482
- PMCID: PMC2635034
- DOI: 10.1074/jbc.M806987200
Two arginine-glutamate ionic locks near the extracellular surface of FFAR1 gate receptor activation
Abstract
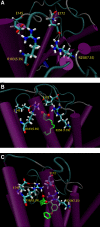
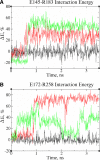
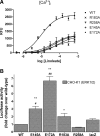
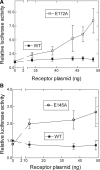
Activation of a number of class A G protein-coupled receptors (GPCRs) is thought to involve two molecular switches, a rotamer toggle switch within the transmembrane domain and an ionic lock at the cytoplasmic surface of the receptor; however, the mechanism by which agonist binding changes these molecular interactions is not understood. Importantly, 80% of GPCRs including free fatty acid receptor 1 (FFAR1) lack the complement of amino acid residues implicated in either or both of these two switches; the mechanism of activation of these GPCRs is therefore less clear. By homology modeling, we identified two Glu residues (Glu-145 and Glu-172) in the second extracellular loop of FFAR1 that form putative interactions individually with two transmembrane Arg residues (Arg-183(5.39) and Arg-258(7.35)) to create two ionic locks. Molecular dynamics simulations showed that binding of agonists to FFAR1 leads to breakage of these Glu-Arg interactions. In mutagenesis experiments, breakage of these two putative interactions by substituting Ala for Glu-145 and Glu-172 caused constitutive receptor activation. Our results therefore reveal a molecular switch for receptor activation present on the extracellular surface of FFAR1 that is broken by agonist binding. Similar ionic locks between the transmembrane domains and the extracellular loops may constitute a mechanism common to other class A GPCRs also.
Figures

Similar articles
-
Charged extracellular residues, conserved throughout a G-protein-coupled receptor family, are required for ligand binding, receptor activation, and cell-surface expression.J Biol Chem. 2006 Dec 15;281(50):38478-88. doi: 10.1074/jbc.M607639200. Epub 2006 Sep 21. J Biol Chem. 2006. PMID: 16990262
-
Agonist-induced conformational changes in bovine rhodopsin: insight into activation of G-protein-coupled receptors.J Mol Biol. 2008 Oct 3;382(2):539-55. doi: 10.1016/j.jmb.2008.06.084. Epub 2008 Jul 7. J Mol Biol. 2008. PMID: 18638482
-
Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6.J Biol Chem. 2001 Aug 3;276(31):29171-7. doi: 10.1074/jbc.M103747200. Epub 2001 May 25. J Biol Chem. 2001. PMID: 11375997
-
Action of molecular switches in GPCRs--theoretical and experimental studies.Curr Med Chem. 2012;19(8):1090-109. doi: 10.2174/092986712799320556. Curr Med Chem. 2012. PMID: 22300046 Free PMC article. Review.
-
Molecular switches in GPCRs.Curr Opin Struct Biol. 2019 Apr;55:114-120. doi: 10.1016/j.sbi.2019.03.017. Epub 2019 May 10. Curr Opin Struct Biol. 2019. PMID: 31082695 Review.
Cited by
-
Extracellular loop 2 of the free fatty acid receptor 2 mediates allosterism of a phenylacetamide ago-allosteric modulator.Mol Pharmacol. 2011 Jul;80(1):163-73. doi: 10.1124/mol.110.070789. Epub 2011 Apr 15. Mol Pharmacol. 2011. PMID: 21498659 Free PMC article.
-
Molecular mechanism of fatty acid activation of FFAR1.Proc Natl Acad Sci U S A. 2023 May 30;120(22):e2219569120. doi: 10.1073/pnas.2219569120. Epub 2023 May 22. Proc Natl Acad Sci U S A. 2023. PMID: 37216523 Free PMC article.
-
Docking-based virtual screening for ligands of G protein-coupled receptors: not only crystal structures but also in silico models.J Mol Graph Model. 2011 Feb;29(5):614-23. doi: 10.1016/j.jmgm.2010.11.005. Epub 2010 Nov 19. J Mol Graph Model. 2011. PMID: 21146435 Free PMC article.
-
Molecular mechanisms of target recognition by lipid GPCRs: relevance for cancer.Oncogene. 2016 Aug 4;35(31):4021-35. doi: 10.1038/onc.2015.467. Epub 2015 Dec 7. Oncogene. 2016. PMID: 26640151 Review.
-
In-Vivo Antidiabetic Activity and In-Silico Mode of Action of LC/MS-MS Identified Flavonoids in Oleaster Leaves.Molecules. 2020 Nov 1;25(21):5073. doi: 10.3390/molecules25215073. Molecules. 2020. PMID: 33139638 Free PMC article.
References
-
- Cristalli, G., Lambertucci, C., Marucci, G., Volpini, R., and Dal Ben, D. (2008) Curr. Pharm. Des. 14 1525–1552 - PubMed
-
- Lagerström, M. C., and Schiöth, H. B. (2008) Nat. Rev. Drug. Discov. 7 339–357 - PubMed
-
- Shi, L., Liapakis, G., Xu, R., Guarnieri, F., Ballesteros, J. A., and Javitch, J. A. (2002) J. Biol. Chem. 277 40989–40996 - PubMed
-
- Ballesteros, J. A., Jensen, A. D., Liapakis, G., Rasmussen, S. G., Shi, L., Gether, U., and Javitch, J. A. (2001) J. Biol. Chem. 276 29171–29177 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

