ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development
- PMID: 19060883
- PMCID: PMC2642523
- DOI: 10.1038/nature07575
ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development
Erratum in
- Nature. 2009 Mar 26;458(7237):538
Abstract
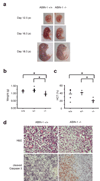
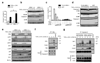
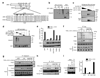
Proteins that directly regulate tumour necrosis factor receptor (TNFR) signalling have critical roles in regulating cellular activation and survival. ABIN-1 (A20 binding and inhibitor of NF-kappaB) is a novel protein that is thought to inhibit NF-kappaB signalling. Here we show that mice deficient for ABIN-1 die during embryogenesis with fetal liver apoptosis, anaemia and hypoplasia. ABIN-1 deficient cells are hypersensitive to tumour necrosis factor (TNF)-induced programmed cell death, and TNF deficiency rescues ABIN-1 deficient embryos. ABIN-1 inhibits caspase 8 recruitment to FADD (Fas-associated death domain-containing protein) in TNF-induced signalling complexes, preventing caspase 8 cleavage and programmed cell death. Moreover, ABIN-1 directly binds polyubiquitin chains and this ubiquitin sensing activity is required for ABIN-1's anti-apoptotic activity. These studies provide insights into how ubiquitination and ubiquitin sensing proteins regulate cellular and organismal survival.
Figures
Similar articles
-
ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling.Biochem Pharmacol. 2009 Jul 15;78(2):105-14. doi: 10.1016/j.bcp.2009.02.009. Epub 2009 Feb 27. Biochem Pharmacol. 2009. PMID: 19464428 Review.
-
The biology of A20-binding inhibitors of NF-kappaB activation (ABINs).Adv Exp Med Biol. 2014;809:13-31. doi: 10.1007/978-1-4939-0398-6_2. Adv Exp Med Biol. 2014. PMID: 25302363 Review.
-
ABIN-1 is a key regulator in RIPK1-dependent apoptosis (RDA) and necroptosis, and ABIN-1 deficiency potentiates necroptosis-based cancer therapy in colorectal cancer.Cell Death Dis. 2021 Feb 1;12(2):140. doi: 10.1038/s41419-021-03427-y. Cell Death Dis. 2021. PMID: 33542218 Free PMC article.
-
Identification of a novel A20-binding inhibitor of nuclear factor-kappa B activation termed ABIN-2.J Biol Chem. 2001 Aug 10;276(32):30216-23. doi: 10.1074/jbc.M100048200. Epub 2001 Jun 4. J Biol Chem. 2001. PMID: 11390377
-
LIND/ABIN-3 is a novel lipopolysaccharide-inducible inhibitor of NF-kappaB activation.J Biol Chem. 2007 Jan 5;282(1):81-90. doi: 10.1074/jbc.M607481200. Epub 2006 Nov 6. J Biol Chem. 2007. PMID: 17088249
Cited by
-
Downregulation of TNIP1 Expression Leads to Increased Proliferation of Human Keratinocytes and Severer Psoriasis-Like Conditions in an Imiquimod-Induced Mouse Model of Dermatitis.PLoS One. 2015 Jun 5;10(6):e0127957. doi: 10.1371/journal.pone.0127957. eCollection 2015. PLoS One. 2015. PMID: 26046540 Free PMC article.
-
TLR7 Protein Expression in Mild and Severe Lupus-Prone Models Is Regulated in a Leukocyte, Genetic, and IRAK4 Dependent Manner.Front Immunol. 2019 Jul 10;10:1546. doi: 10.3389/fimmu.2019.01546. eCollection 2019. Front Immunol. 2019. PMID: 31354711 Free PMC article.
-
Deubiquitinases in the regulation of NF-κB signaling.Cell Res. 2011 Jan;21(1):22-39. doi: 10.1038/cr.2010.166. Epub 2010 Nov 30. Cell Res. 2011. PMID: 21119682 Free PMC article. Review.
-
Signaling Crosstalk Mechanisms That May Fine-Tune Pathogen-Responsive NFκB.Front Immunol. 2019 Jul 2;10:433. doi: 10.3389/fimmu.2019.00433. eCollection 2019. Front Immunol. 2019. PMID: 31312197 Free PMC article. Review.
-
Met1-linked ubiquitin signalling in health and disease: inflammation, immunity, cancer, and beyond.Cell Death Differ. 2021 Feb;28(2):473-492. doi: 10.1038/s41418-020-00676-w. Epub 2021 Jan 13. Cell Death Differ. 2021. PMID: 33441937 Free PMC article. Review.
References
-
- Heyninck K, De Valck D, Vanden Berghe W, Van Criekinge W, Contreras R, Fiers W, Haegeman G, Beyaert R. The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN-1. J Cell Biol. 1999;145:1471–1482. - PMC - PubMed
-
- Opipari AW, Jr, Boguski MS, Dixit VM. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J Biol Chem. 1990;265:14705–14708. - PubMed
-
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. - PubMed
-
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

