Wnt-reporter expression pattern in the mouse intestine during homeostasis
- PMID: 19055726
- PMCID: PMC2615026
- DOI: 10.1186/1471-230X-8-57
Wnt-reporter expression pattern in the mouse intestine during homeostasis
Abstract
Background: The canonical Wnt signaling pathway is a known regulator of cell proliferation during development and maintenance of the intestinal epithelium. Perturbations in this pathway lead to aberrant epithelial proliferation and intestinal cancer. In the mature intestine, proliferation is confined to the relatively quiescent stem cells and the rapidly cycling transient-amplifying cells in the intestinal crypts. Although the Wnt signal is believed to regulate all proliferating intestinal cells, surprisingly, this has not been thoroughly demonstrated. This important determination has implications on intestinal function, especially during epithelial expansion and regeneration, and warrants an extensive characterization of Wnt-activated cells.
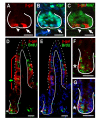
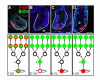
Methods: To identify intestinal epithelial cells that actively receive a Wnt signal, we analyzed intestinal Wnt-reporter expression patterns in two different mouse lines using immunohistochemistry, enzymatic activity, in situ hybridization and qRT-PCR, then corroborated results with reporter-independent analyses. Wnt-receiving cells were further characterized for co-expression of proliferation markers, putative stem cell markers and cellular differentiation markers using an immunohistochemical approach. Finally, to demonstrate that Wnt-reporter mice have utility in detecting perturbations in intestinal Wnt signaling, the reporter response to gamma-irradiation was examined.
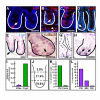
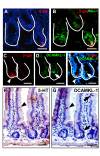
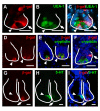
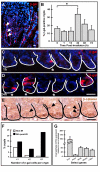
Results: Wnt-activated cells were primarily restricted to the base of the small intestinal and colonic crypts, and were highest in numbers in the proximal small intestine, decreasing in frequency in a gradient toward the large intestine. Interestingly, the majority of the Wnt-reporter-expressing cells did not overlap with the transient-amplifying cell population. Further, while Wnt-activated cells expressed the putative stem cell marker Musashi-1, they did not co-express DCAMKL-1 or cell differentiation markers. Finally, gamma-irradiation stimulated an increase in Wnt-activated intestinal crypt cells.
Conclusion: We show, for the first time, detailed characterization of the intestine from Wnt-reporter mice. Further, our data show that the majority of Wnt-receiving cells reside in the stem cell niche of the crypt base and do not extend into the proliferative transient-amplifying cell population. We also show that the Wnt-reporter mice can be used to detect changes in intestinal epithelial Wnt signaling upon physiologic injury. Our findings have an important impact on understanding the regulation of the intestinal stem cell hierarchy during homeostasis and in disease states.
Figures
Similar articles
-
Phases of canonical Wnt signaling during the development of mouse intestinal epithelium.Gastroenterology. 2007 Aug;133(2):529-38. doi: 10.1053/j.gastro.2007.04.072. Epub 2007 May 3. Gastroenterology. 2007. PMID: 17681174
-
Loss of caveolin-1 causes the hyper-proliferation of intestinal crypt stem cells, with increased sensitivity to whole body gamma-radiation.Cell Cycle. 2005 Dec;4(12):1817-25. doi: 10.4161/cc.4.12.2199. Epub 2005 Dec 22. Cell Cycle. 2005. PMID: 16294037
-
Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract.Gastroenterology. 2010 Dec;139(6):2072-2082.e5. doi: 10.1053/j.gastro.2010.08.053. Epub 2010 Oct 19. Gastroenterology. 2010. PMID: 20826154 Free PMC article.
-
Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche.Transl Res. 2010 Sep;156(3):180-7. doi: 10.1016/j.trsl.2010.06.003. Epub 2010 Jul 3. Transl Res. 2010. PMID: 20801415 Free PMC article. Review.
-
The gastrointestinal tract stem cell niche.Stem Cell Rev. 2006;2(3):203-12. doi: 10.1007/s12015-006-0048-1. Stem Cell Rev. 2006. PMID: 17625256 Review.
Cited by
-
Cell organisation in the colonic crypt: a theoretical comparison of the pedigree and niche concepts.PLoS One. 2013 Sep 12;8(9):e73204. doi: 10.1371/journal.pone.0073204. eCollection 2013. PLoS One. 2013. PMID: 24069177 Free PMC article.
-
A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse.BMC Dev Biol. 2010 Dec 21;10:121. doi: 10.1186/1471-213X-10-121. BMC Dev Biol. 2010. PMID: 21176145 Free PMC article.
-
Safety Considerations in the Development of Hippo Pathway Inhibitors in Cancers.Front Cell Dev Biol. 2019 Aug 14;7:156. doi: 10.3389/fcell.2019.00156. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31475147 Free PMC article. Review.
-
Arid1a is essential for intestinal stem cells through Sox9 regulation.Proc Natl Acad Sci U S A. 2019 Jan 29;116(5):1704-1713. doi: 10.1073/pnas.1804858116. Epub 2019 Jan 11. Proc Natl Acad Sci U S A. 2019. PMID: 30635419 Free PMC article.
-
Combined inadequacies of multiple B vitamins amplify colonic Wnt signaling and promote intestinal tumorigenesis in BAT-LacZxApc1638N mice.FASEB J. 2011 Sep;25(9):3136-45. doi: 10.1096/fj.11-184143. Epub 2011 Jun 6. FASEB J. 2011. PMID: 21646397 Free PMC article.
References
-
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

