Human papillomavirus E7 protein deregulates mitosis via an association with nuclear mitotic apparatus protein 1
- PMID: 19052088
- PMCID: PMC2643759
- DOI: 10.1128/JVI.01971-08
Human papillomavirus E7 protein deregulates mitosis via an association with nuclear mitotic apparatus protein 1
Abstract
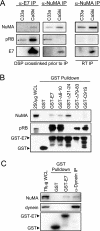
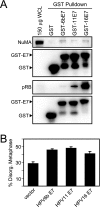
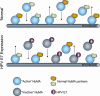
We previously observed that high-risk human papillomavirus type 16 (HPV16) E7 expression leads to the delocalization of dynein from mitotic spindles (C. L. Nguyen, M. E. McLaughlin-Drubin, and K. Munger, Cancer Res. 68:8715-8722, 2008). Here, we show that HPV16 E7 associates with nuclear mitotic apparatus protein 1 (NuMA) and that NuMA binding and the ability to induce dynein delocalization map to similar carboxyl-terminal sequences of E7. Additionally, we show that the delocalization of dynein from mitotic spindles by HPV16 E7 and the interaction between HPV16 E7 and NuMA correlate with the induction of defects in chromosome alignment during prometaphase even in cells with normal centrosome numbers. Furthermore, low-risk HPV6b and HPV11 E7s also associate with NuMA and also induce a similar mitotic defect. It is possible that the disruption of mitotic events by HPV E7, via targeting of the NuMA/dynein complex and potentially other NuMA-containing complexes, contributes to viral maintenance and propagation potentially through abrogating the differentiation program of the infected epithelium. Furthermore, in concert with activities specific to high-risk HPV E6 and E7, such as the inactivation of the p53 and pRB tumor suppressors, respectively, the disruption of the NuMA/dynein network may result in mitotic errors that would make an infected cell more prone to the accumulation of aneuploidy even in the absence of supernumerary centrosomes.
Figures



Similar articles
-
Delocalization of the microtubule motor Dynein from mitotic spindles by the human papillomavirus E7 oncoprotein is not sufficient for induction of multipolar mitoses.Cancer Res. 2008 Nov 1;68(21):8715-22. doi: 10.1158/0008-5472.CAN-08-1303. Cancer Res. 2008. PMID: 18974113 Free PMC article.
-
BetaHPV E6 and E7 colocalize with NuMa in dividing keratinocytes.Virus Genes. 2019 Oct;55(5):600-609. doi: 10.1007/s11262-019-01685-9. Epub 2019 Jul 9. Virus Genes. 2019. PMID: 31290065
-
Nuclear Mitotic Apparatus (NuMA) Interacts with and Regulates Astrin at the Mitotic Spindle.J Biol Chem. 2016 Sep 16;291(38):20055-67. doi: 10.1074/jbc.M116.724831. Epub 2016 Jul 26. J Biol Chem. 2016. PMID: 27462074 Free PMC article.
-
Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein.Front Biosci. 2006 Jan 1;11:1137-46. doi: 10.2741/1868. Front Biosci. 2006. PMID: 16146802 Review.
-
NuMA: a nuclear protein involved in mitotic centrosome function.Microsc Res Tech. 2000 Jun 1;49(5):467-77. doi: 10.1002/(SICI)1097-0029(20000601)49:5<467::AID-JEMT9>3.0.CO;2-V. Microsc Res Tech. 2000. PMID: 10842374 Review.
Cited by
-
Mitotic control of human papillomavirus genome-containing cells is regulated by the function of the PDZ-binding motif of the E6 oncoprotein.Oncotarget. 2017 Mar 21;8(12):19491-19506. doi: 10.18632/oncotarget.14469. Oncotarget. 2017. PMID: 28061478 Free PMC article.
-
Role of prolonged mitotic checkpoint activation in the formation and treatment of cancer.Future Oncol. 2009 Nov;5(9):1363-70. doi: 10.2217/fon.09.118. Future Oncol. 2009. PMID: 19903065 Free PMC article. Review.
-
Tight junction protein occludin regulates progenitor Self-Renewal and survival in developing cortex.Elife. 2019 Dec 3;8:e49376. doi: 10.7554/eLife.49376. Elife. 2019. PMID: 31794381 Free PMC article.
-
Chlamydia trachomatis infection causes mitotic spindle pole defects independently from its effects on centrosome amplification.Traffic. 2011 Jul;12(7):854-66. doi: 10.1111/j.1600-0854.2011.01204.x. Epub 2011 May 12. Traffic. 2011. PMID: 21477082 Free PMC article.
-
Genomic Instability Induced By Human Papillomavirus Oncogenes.N Am J Med Sci (Boston). 2010 Apr;3(2):43-47. doi: 10.7156/v3i2p043. N Am J Med Sci (Boston). 2010. PMID: 21643539 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

