Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila
- PMID: 19043549
- PMCID: PMC2582680
- DOI: 10.1371/journal.ppat.1000220
Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila
Abstract
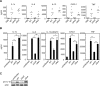
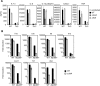
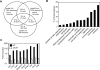
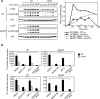
The immune system must discriminate between pathogenic and nonpathogenic microbes in order to initiate an appropriate response. Toll-like receptors (TLRs) detect microbial components common to both pathogenic and nonpathogenic bacteria, whereas Nod-like receptors (NLRs) sense microbial components introduced into the host cytosol by the specialized secretion systems or pore-forming toxins of bacterial pathogens. The host signaling pathways that respond to bacterial secretion systems remain poorly understood. Infection with the pathogen Legionella pneumophila, which utilizes a type IV secretion system (T4SS), induced an increased proinflammatory cytokine response compared to avirulent bacteria in which the T4SS was inactivated. This enhanced response involved NF-kappaB activation by TLR signaling as well as Nod1 and Nod2 detection of type IV secretion. Furthermore, a TLR- and RIP2-independent pathway leading to p38 and SAPK/JNK MAPK activation was found to play an equally important role in the host response to virulent L. pneumophila. Activation of this MAPK pathway was T4SS-dependent and coordinated with TLR signaling to mount a robust proinflammatory cytokine response to virulent L. pneumophila. These findings define a previously uncharacterized host response to bacterial type IV secretion that activates MAPK signaling and demonstrate that coincident detection of multiple bacterial components enables immune discrimination between virulent and avirulent bacteria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Molecular characterization of Legionella pneumophila-induced interleukin-8 expression in T cells.BMC Microbiol. 2010 Jan 5;10:1. doi: 10.1186/1471-2180-10-1. BMC Microbiol. 2010. Retraction in: BMC Microbiol. 2011 Jun 02;11:127. doi: 10.1186/1471-2180-11-127. PMID: 20051107 Free PMC article. Retracted.
-
Induction of human β-defensin-2 in pulmonary epithelial cells by Legionella pneumophila: involvement of TLR2 and TLR5, p38 MAPK, JNK, NF-κB, and AP-1.Am J Physiol Lung Cell Mol Physiol. 2010 May;298(5):L687-95. doi: 10.1152/ajplung.00365.2009. Epub 2010 Feb 12. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20154223
-
The Type II Secretion System of Legionella pneumophila Dampens the MyD88 and Toll-Like Receptor 2 Signaling Pathway in Infected Human Macrophages.Infect Immun. 2017 Mar 23;85(4):e00897-16. doi: 10.1128/IAI.00897-16. Print 2017 Apr. Infect Immun. 2017. PMID: 28138020 Free PMC article.
-
Inflammasomes, Autophagy, and Cell Death: The Trinity of Innate Host Defense against Intracellular Bacteria.Mediators Inflamm. 2019 Jan 8;2019:2471215. doi: 10.1155/2019/2471215. eCollection 2019. Mediators Inflamm. 2019. PMID: 30728749 Free PMC article. Review.
-
Intracellular NOD-like receptors in host defense and disease.Immunity. 2007 Oct;27(4):549-59. doi: 10.1016/j.immuni.2007.10.002. Immunity. 2007. PMID: 17967410 Review.
Cited by
-
Immunology taught by bacteria.J Clin Immunol. 2010 Jul;30(4):507-11. doi: 10.1007/s10875-010-9389-2. Epub 2010 Apr 6. J Clin Immunol. 2010. PMID: 20373001 Free PMC article. Review.
-
Viewing Legionella pneumophila Pathogenesis through an Immunological Lens.J Mol Biol. 2019 Oct 4;431(21):4321-4344. doi: 10.1016/j.jmb.2019.07.028. Epub 2019 Jul 25. J Mol Biol. 2019. PMID: 31351897 Free PMC article. Review.
-
Legionnaires' Disease Mortality in Guinea Pigs Involves the p45 Mobile Genomic Element.J Infect Dis. 2019 Oct 8;220(10):1700-1710. doi: 10.1093/infdis/jiz340. J Infect Dis. 2019. PMID: 31268152 Free PMC article.
-
Immune Control of Legionella Infection: An in vivo Perspective.Front Microbiol. 2011 Jun 3;2:126. doi: 10.3389/fmicb.2011.00126. eCollection 2011. Front Microbiol. 2011. PMID: 21687433 Free PMC article.
-
Primary Role for Toll-Like Receptor-Driven Tumor Necrosis Factor Rather than Cytosolic Immune Detection in Restricting Coxiella burnetii Phase II Replication within Mouse Macrophages.Infect Immun. 2016 Mar 24;84(4):998-1015. doi: 10.1128/IAI.01536-15. Print 2016 Apr. Infect Immun. 2016. PMID: 26787725 Free PMC article.
References
-
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54 Pt 1:1–13. - PubMed
-
- Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens. Nature. 2007;449:827–834. - PubMed
-
- Inohara, Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. - PubMed
-
- Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. - PubMed
-
- Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

