Dictyostelium Dock180-related RacGEFs regulate the actin cytoskeleton during cell motility
- PMID: 19037099
- PMCID: PMC2626555
- DOI: 10.1091/mbc.e08-09-0899
Dictyostelium Dock180-related RacGEFs regulate the actin cytoskeleton during cell motility
Abstract
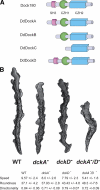
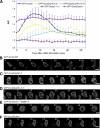
Cell motility of amoeboid cells is mediated by localized F-actin polymerization that drives the extension of membrane protrusions to promote forward movements. We show that deletion of either of two members of the Dictyostelium Dock180 family of RacGEFs, DockA and DockD, causes decreased speed of chemotaxing cells. The phenotype is enhanced in the double mutant and expression of DockA or DockD complements the reduced speed of randomly moving DockD null cells' phenotype, suggesting that DockA and DockD are likely to act redundantly and to have similar functions in regulating cell movement. In this regard, we find that overexpressing DockD causes increased cell speed by enhancing F-actin polymerization at the sites of pseudopod extension. DockD localizes to the cell cortex upon chemoattractant stimulation and at the leading edge of migrating cells and this localization is dependent on PI3K activity, suggesting that DockD might be part of the pathway that links PtdIns(3,4,5)P(3) production to F-actin polymerization. Using a proteomic approach, we found that DdELMO1 is associated with DockD and that Rac1A and RacC are possible in vivo DockD substrates. In conclusion, our work provides a further understanding of how cell motility is controlled and provides evidence that the molecular mechanism underlying Dock180-related protein function is evolutionarily conserved.
Figures





Similar articles
-
Rac regulation of chemotaxis and morphogenesis in Dictyostelium.EMBO J. 2004 Oct 27;23(21):4177-89. doi: 10.1038/sj.emboj.7600368. Epub 2004 Oct 7. EMBO J. 2004. PMID: 15470506 Free PMC article.
-
A shortcut from GPCR signaling to Rac-mediated actin cytoskeleton through an ELMO/DOCK complex.Small GTPases. 2012 Jul-Sep;3(3):183-5. doi: 10.4161/sgtp.20271. Epub 2012 May 31. Small GTPases. 2012. PMID: 22647486 Free PMC article.
-
Assaying Rho GTPase-Dependent Processes in Dictyostelium discoideum.Methods Mol Biol. 2018;1821:371-392. doi: 10.1007/978-1-4939-8612-5_25. Methods Mol Biol. 2018. PMID: 30062425
-
Molecular analysis of racE function in Dictyostelium.Microsc Res Tech. 2000 Apr 15;49(2):145-51. doi: 10.1002/(SICI)1097-0029(20000415)49:2<145::AID-JEMT6>3.0.CO;2-A. Microsc Res Tech. 2000. PMID: 10816253 Review.
-
Dictyostelium as model system for studies of the actin cytoskeleton by molecular genetics.Microsc Res Tech. 1999 Oct 15;47(2):124-34. doi: 10.1002/(SICI)1097-0029(19991015)47:2<124::AID-JEMT5>3.0.CO;2-8. Microsc Res Tech. 1999. PMID: 10523791 Review.
Cited by
-
Zizimin and Dock guanine nucleotide exchange factors in cell function and disease.Small GTPases. 2013 Jan-Mar;4(1):22-7. doi: 10.4161/sgtp.22087. Epub 2012 Dec 17. Small GTPases. 2013. PMID: 23247359 Free PMC article.
-
Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity.Annu Rev Biophys. 2010;39:265-89. doi: 10.1146/annurev.biophys.093008.131228. Annu Rev Biophys. 2010. PMID: 20192768 Free PMC article. Review.
-
The novel RacE-binding protein GflB sharpens Ras activity at the leading edge of migrating cells.Mol Biol Cell. 2016 May 15;27(10):1596-605. doi: 10.1091/mbc.E15-11-0796. Epub 2016 Mar 23. Mol Biol Cell. 2016. PMID: 27009206 Free PMC article.
-
Pleiotropic roles of a ribosomal protein in Dictyostelium discoideum.PLoS One. 2012;7(2):e30644. doi: 10.1371/journal.pone.0030644. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363460 Free PMC article.
-
Stochastic Dynamics of Membrane Protrusion Mediated by the DOCK180/Rac Pathway in Migrating Cells.Cell Mol Bioeng. 2010 Mar 1;3(1):30-39. doi: 10.1007/s12195-010-0100-8. Cell Mol Bioeng. 2010. PMID: 20473365 Free PMC article.
References
-
- Bianco A., Poukkula M., Cliffe A., Mathieu J., Luque C. M., Fulga T. A., Rorth P. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 2007;448:362–365. - PubMed
-
- Brugnera E., Haney L., Grimsley C., Lu M., Walk S. F., Tosello-Trampont A. C., Macara I. G., Madhani H., Fink G. R., Ravichandran K. S. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 2002;4:574–582. - PubMed
-
- Côté J., Vuori K., William E., Balch C.J.D., Alan H. Methods in Enzymology. Vol. 406. New York: Academic Press; 2006. In vitro guanine nucleotide exchange activity of DHR:]2/DOCKER/CZH2 domains; pp. 41–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

