Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans
- PMID: 19033672
- PMCID: PMC2582442
- DOI: 10.1172/JCI36264
Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans
Abstract
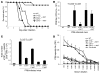
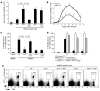
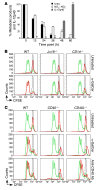
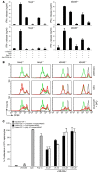
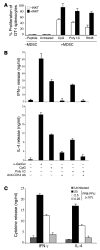
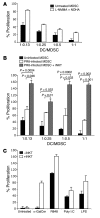
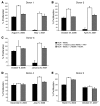
Infection with influenza A virus (IAV) presents a substantial threat to public health worldwide, with young, elderly, and immunodeficient individuals being particularly susceptible. Inflammatory responses play an important role in the fatal outcome of IAV infection, but the mechanism remains unclear. We demonstrate here that the absence of invariant NKT (iNKT) cells in mice during IAV infection resulted in the expansion of myeloid-derived suppressor cells (MDSCs), which suppressed IAV-specific immune responses through the expression of both arginase and NOS, resulting in high IAV titer and increased mortality. Adoptive transfer of iNKT cells abolished the suppressive activity of MDSCs, restored IAV-specific immune responses, reduced IAV titer, and increased survival rate. The crosstalk between iNKT and MDSCs was CD1d- and CD40-dependent. Furthermore, IAV infection and exposure to TLR agonists relieved the suppressive activity of MDSCs. Finally, we extended these results to humans by demonstrating the presence of myeloid cells with suppressive activity in the PBLs of individuals infected with IAV and showed that their suppressive activity is substantially reduced by iNKT cell activation. These findings identify what we believe to be a novel immunomodulatory role of iNKT cells, which we suggest could be harnessed to abolish the immunosuppressive activity of MDSCs during IAV infection.
Figures
Similar articles
-
Activated invariant NKT cells control central nervous system autoimmunity in a mechanism that involves myeloid-derived suppressor cells.J Immunol. 2013 Mar 1;190(5):1948-60. doi: 10.4049/jimmunol.1201718. Epub 2013 Jan 23. J Immunol. 2013. PMID: 23345328 Free PMC article.
-
On/off TLR signaling decides proinflammatory or tolerogenic dendritic cell maturation upon CD1d-mediated interaction with invariant NKT cells.J Immunol. 2010 Dec 15;185(12):7317-29. doi: 10.4049/jimmunol.1000400. Epub 2010 Nov 15. J Immunol. 2010. PMID: 21078913
-
Tumor-induced CD11b(+) Gr-1(+) myeloid-derived suppressor cells exacerbate immune-mediated hepatitis in mice in a CD40-dependent manner.Eur J Immunol. 2015 Apr;45(4):1148-58. doi: 10.1002/eji.201445093. Epub 2015 Feb 23. Eur J Immunol. 2015. PMID: 25616156 Free PMC article.
-
Invariant NKT cells: regulation and function during viral infection.PLoS Pathog. 2012;8(8):e1002838. doi: 10.1371/journal.ppat.1002838. Epub 2012 Aug 16. PLoS Pathog. 2012. PMID: 22916008 Free PMC article. Review.
-
Modulation of Immune Responses to Influenza A Virus Vaccines by Natural Killer T Cells.Front Immunol. 2020 Oct 20;11:2172. doi: 10.3389/fimmu.2020.02172. eCollection 2020. Front Immunol. 2020. PMID: 33193296 Free PMC article. Review.
Cited by
-
Immune Suppression by Myeloid Cells in HIV Infection: New Targets for Immunotherapy.Open AIDS J. 2014 Dec 29;8:66-78. doi: 10.2174/1874613601408010066. eCollection 2014. Open AIDS J. 2014. PMID: 25624956 Free PMC article.
-
Expression patterns of the immunomodulatory enzyme arginase 1 in blood, lymph nodes and tumor tissue of early-stage breast cancer patients.Oncoimmunology. 2012 Nov 1;1(8):1305-1312. doi: 10.4161/onci.21678. Oncoimmunology. 2012. PMID: 23243594 Free PMC article.
-
Nonclassical MHC-Restricted Invariant Vα6 T Cells Are Critical for Efficient Early Innate Antiviral Immunity in the Amphibian Xenopus laevis.J Immunol. 2015 Jul 15;195(2):576-86. doi: 10.4049/jimmunol.1500458. Epub 2015 Jun 10. J Immunol. 2015. PMID: 26062996 Free PMC article.
-
Invariant natural killer T cells as sensors and managers of inflammation.Trends Immunol. 2013 Feb;34(2):50-8. doi: 10.1016/j.it.2012.08.009. Epub 2012 Sep 25. Trends Immunol. 2013. PMID: 23017731 Free PMC article. Review.
-
Recent advances in myeloid-derived suppressor cell biology.Front Med. 2021 Apr;15(2):232-251. doi: 10.1007/s11684-020-0797-2. Epub 2020 Sep 2. Front Med. 2021. PMID: 32876877 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

