Sex differences in histone modifications in the neonatal mouse brain
- PMID: 19029819
- PMCID: PMC2667098
- DOI: 10.4161/epi.4.1.7288
Sex differences in histone modifications in the neonatal mouse brain
Abstract
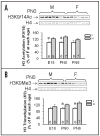
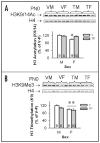
Sex differences in neural development are established via a number of cellular processes (i.e., migration, death and survival). One critical factor identified is the neonatal rise in testosterone (T) which activates gene transcription via androgen (AR) and, after aromatization to estradiol, estrogen receptors (ERalpha and beta). Recent evidence shows that AR and ERs interact with histone modifying enzymes. Post-translational modifications of histones, including acetylation and methylation, are involved in transcriptional regulation during normal development. Therefore, we hypothesized that acetylation and/or methylation of histone H3 may underlie sexual differentiation, at least in some regions of the brain. We measured levels of acetylated (H3K9/14Ac) and trimethylated (H3K9Me3) H3 in whole neonatal mouse brains and in three regions: preoptic area + hypothalamus, amygdala and cortex + hippocampus (CTX/HIP). Sex differences in H3K9/14Ac and H3K9Me3 (males > females) were noted in the CTX/HIP on embryonic day 18, the day of birth, and six days later. To determine if T mediates these changes in H3 modifications, pregnant dams received vehicle or T for the final four days of gestation; pup brains were collected at birth. Methylation of H3 was sexually dimorphic despite hormone treatment. In contrast, H3 acetylation in the CTX/HIP of females from T-treated dams rose to levels equivalent to males. Thus, H3 modifications are sexually dimorphic in the developing mouse CTX/HIP and acetylation, but not methylation, is masculinized in females by T in utero. This is the first demonstration that histone modification is associated with neural sexual differentiation.
Figures

Similar articles
-
Sex-dependent effects of lead and prenatal stress on post-translational histone modifications in frontal cortex and hippocampus in the early postnatal brain.Neurotoxicology. 2016 May;54:65-71. doi: 10.1016/j.neuro.2016.03.016. Epub 2016 Mar 24. Neurotoxicology. 2016. PMID: 27018513 Free PMC article.
-
Epigenetic control of translation regulation: alterations in histone H3 lysine 9 post-translation modifications are correlated with the expression of the translation initiation factor 2B (Eif2b5) during thermal control establishment.Dev Neurobiol. 2010 Feb;70(2):100-13. doi: 10.1002/dneu.20763. Dev Neurobiol. 2010. PMID: 19950192
-
Developmental exposure to 50 parts-per-billion arsenic influences histone modifications and associated epigenetic machinery in a region- and sex-specific manner in the adult mouse brain.Toxicol Appl Pharmacol. 2015 Oct 1;288(1):40-51. doi: 10.1016/j.taap.2015.07.013. Epub 2015 Jul 17. Toxicol Appl Pharmacol. 2015. PMID: 26193056 Free PMC article.
-
Epigenetic landscape of amphetamine and methamphetamine addiction in rodents.Epigenetics. 2015;10(7):574-80. doi: 10.1080/15592294.2015.1055441. Epigenetics. 2015. PMID: 26023847 Free PMC article. Review.
-
Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility.Prog Brain Res. 2010;186:77-95. doi: 10.1016/B978-0-444-53630-3.00006-3. Prog Brain Res. 2010. PMID: 21094887 Free PMC article. Review.
Cited by
-
Sex-Bias in Irritable Bowel Syndrome: Linking Steroids to the Gut-Brain Axis.Front Endocrinol (Lausanne). 2021 May 19;12:684096. doi: 10.3389/fendo.2021.684096. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34093447 Free PMC article. Review.
-
Sex differences in Gadd45b expression and methylation in the developing rodent amygdala.Brain Res. 2016 Jul 1;1642:461-466. doi: 10.1016/j.brainres.2016.04.031. Epub 2016 Apr 14. Brain Res. 2016. PMID: 27086974 Free PMC article.
-
What a difference an X or Y makes: sex chromosomes, gene dose, and epigenetics in sexual differentiation.Handb Exp Pharmacol. 2012;(214):67-88. doi: 10.1007/978-3-642-30726-3_4. Handb Exp Pharmacol. 2012. PMID: 23027446 Free PMC article. Review.
-
Sex differences in DNA methylation may contribute to risk of PTSD and depression: a review of existing evidence.Depress Anxiety. 2013 Dec;30(12):1151-60. doi: 10.1002/da.22167. Epub 2013 Aug 19. Depress Anxiety. 2013. PMID: 23959810 Free PMC article. Review.
-
Location, location, location: genetic regulation of neural sex differences.Rev Endocr Metab Disord. 2012 Sep;13(3):151-61. doi: 10.1007/s11154-011-9186-0. Rev Endocr Metab Disord. 2012. PMID: 21607612 Free PMC article. Review.
References
-
- Cheung WL, Briggs SD, Allis CD. Acetylation and chromosomal functions. Curr Opin Cell Biol. 2000;12:326–33. - PubMed
-
- Fukuda S, Taga T. Cell fate determination regulated by a transcriptional signal network in the developing mouse brain. Anat Sci Int. 2005;80:12–8. - PubMed
-
- Li B, Carey M, Workman JL. The Role of Chromatin during Transcription. Cell. 2007;128:707–19. - PubMed
-
- Moore CL, Wong L, Daum MC, Leclair OU. Mother-infant interactions in two strains of rats: implications for dissociating mechanism and function of a maternal pattern. Dev Psychobiol. 1997;30:301–12. - PubMed
-
- Ward IL, Ward OB, Affuso JD, Long WD, French JA, Hendricks SE. Fetal testosterone surge: specific modulations induced in male rats by maternal stress and/or alcohol consumption. Horm Behav. 2003;43:531–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
