Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox
- PMID: 19028840
- PMCID: PMC2660639
- DOI: 10.1096/fj.08-114553
Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox
Abstract
Neutrophils generate microbicidal oxidants through activation of a multicomponent enzyme called NADPH oxidase. During activation, the cytosolic NADPH oxidase components (p47(phox), p67(phox), p40(phox), and Rac2) translocate to the membranes, where they associate with flavocytochrome b(558), which is composed of gp91(phox)/NOX2 and p22(phox), to form the active system. During neutrophil stimulation, p47(phox), p67(phox), p40(phox), and p22(phox) are phosphorylated; however, the phosphorylation of gp91(phox)/NOX2 and its potential role have not been defined. In this study, we show that gp91(phox) is phosphorylated in stimulated neutrophils. The gp91(phox) phosphoprotein is absent in neutrophils from chronic granulomatous disease patients deficient in gp91(phox), which confirms that this phosphoprotein is gp91(phox). The protein kinase C inhibitor GF109203X inhibited phorbol 12-myristate 13-acetate-induced phosphorylation of gp91(phox), and protein kinase C (PKC) phosphorylated the recombinant gp91(phox)- cytosolic carboxy-terminal flavoprotein domain. Two-dimensional tryptic peptide mapping analysis showed that PKC phosphorylated the gp91(phox)-cytosolic tail on the same peptides that were phosphorylated on gp91(phox) in intact cells. In addition, PKC phosphorylation increased diaphorase activity of the gp91(phox) flavoprotein cytosolic domain and its binding to Rac2, p67(phox), and p47(phox). These results demonstrate that gp91(phox) is phosphorylated in human neutrophils by PKC to enhance its catalytic activity and assembly of the complex. Phosphorylation of gp91(phox)/NOX2 is a novel mechanism of NADPH oxidase regulation.
Figures


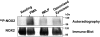
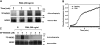

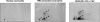
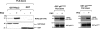
Similar articles
-
The protein kinase A negatively regulates reactive oxygen species production by phosphorylating gp91phox/NOX2 in human neutrophils.Free Radic Biol Med. 2020 Nov 20;160:19-27. doi: 10.1016/j.freeradbiomed.2020.07.021. Epub 2020 Aug 3. Free Radic Biol Med. 2020. PMID: 32758662
-
p40(phox) is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. Implication of a protein kinase c-type kinase in the phosphorylation process.J Biol Chem. 1998 Nov 13;273(46):30097-103. doi: 10.1074/jbc.273.46.30097. J Biol Chem. 1998. PMID: 9804763
-
NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits.Eur J Clin Invest. 2018 Nov;48 Suppl 2:e12951. doi: 10.1111/eci.12951. Epub 2018 Jun 3. Eur J Clin Invest. 2018. PMID: 29757466 Review.
-
A conserved region between the TPR and activation domains of p67phox participates in activation of the phagocyte NADPH oxidase.J Biol Chem. 2010 Oct 8;285(41):31435-45. doi: 10.1074/jbc.M110.161166. Epub 2010 Aug 2. J Biol Chem. 2010. PMID: 20679349 Free PMC article.
-
Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task.Small GTPases. 2014;5:e27952. doi: 10.4161/sgtp.27952. Epub 2014 Mar 5. Small GTPases. 2014. PMID: 24598074 Free PMC article. Review.
Cited by
-
Neutrophil extracellular traps: double-edged swords of innate immunity.J Immunol. 2012 Sep 15;189(6):2689-95. doi: 10.4049/jimmunol.1201719. J Immunol. 2012. PMID: 22956760 Free PMC article. Review.
-
Anvillea garcinii extract inhibits the oxidative burst of primary human neutrophils.BMC Complement Altern Med. 2016 Nov 3;16(1):433. doi: 10.1186/s12906-016-1411-7. BMC Complement Altern Med. 2016. PMID: 27809835 Free PMC article.
-
Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled?Cardiovasc Res. 2010 Jul 15;87(2):281-90. doi: 10.1093/cvr/cvq140. Epub 2010 May 13. Cardiovasc Res. 2010. PMID: 20472564 Free PMC article. Review.
-
Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells.Circ Res. 2010 Apr 30;106(8):1363-73. doi: 10.1161/CIRCRESAHA.109.216036. Epub 2010 Mar 25. Circ Res. 2010. PMID: 20339118 Free PMC article.
-
Emerging evidence for the importance of phosphorylation in the regulation of NADPH oxidases.Antioxid Redox Signal. 2009 Oct;11(10):2429-41. doi: 10.1089/ars.2009.2590. Antioxid Redox Signal. 2009. PMID: 19358632 Free PMC article. Review.
References
-
- Dinauer M C. The respiratory burst oxidase and the molecular genetics of chronic granulomatous disease. Crit Rev Clin Lab Sci. 1993;30:329–369. - PubMed
-
- Chanock S J, El-Benna J, Smith R M, Babior B M. The respiratory burst oxidase. J Biol Chem. 1994;269:24519–24522. - PubMed
-
- Babior B M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64:959–966. - PubMed
-
- Smith J A. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

