Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model
- PMID: 19026746
- PMCID: PMC2741400
- DOI: 10.1016/j.nbd.2008.10.006
Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model
Abstract
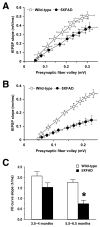
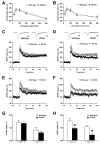
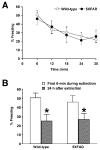
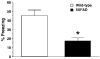
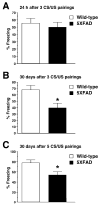
Although animal models of Alzheimer's disease (AD) recapitulate beta-amyloid-dependent hippocampal synaptic and cognitive dysfunctions, it is poorly understood how cortex-dependent remote memory stabilization following initial hippocampal coding is affected. Here, we systematically analyzed biophysical and behavioral phenotypes, including remote memory functions, of 5XFAD APP/PS1 transgenic mice containing five familial AD mutations. We found that 5XFAD mice show hippocampal dysfunctions as observed by reduced levels of baseline transmission and long-term potentiation at Schaffer collateral-CA1 synapses. Hippocampus-dependent memory tested 1 day after contextual fear conditioning was also impaired age-dependently in 5XFAD mice, as correlated with the onset of hippocampal synaptic failures. Importantly, remote memory stabilization during 30 days after training significantly declined in 5XFAD mice at time well before the onset of hippocampal dysfunctions. Our results indicate that 5XFAD mice provide a useful model system to investigate the mechanisms and therapeutic interventions for multiple synaptic and memory dysfunctions associated with AD.
Figures
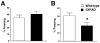
Similar articles
-
Genetic reductions of beta-site amyloid precursor protein-cleaving enzyme 1 and amyloid-beta ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer's disease model mice.Eur J Neurosci. 2010 Jan;31(1):110-8. doi: 10.1111/j.1460-9568.2009.07031.x. Epub 2009 Dec 21. Eur J Neurosci. 2010. PMID: 20092558 Free PMC article.
-
Partial reduction of BACE1 improves synaptic plasticity, recent and remote memories in Alzheimer's disease transgenic mice.J Neurochem. 2010 Apr;113(1):248-61. doi: 10.1111/j.1471-4159.2010.06608.x. Epub 2010 Jan 20. J Neurochem. 2010. PMID: 20089133 Free PMC article.
-
Failures to reconsolidate memory in a mouse model of Alzheimer's disease.Neurobiol Learn Mem. 2009 Oct;92(3):455-9. doi: 10.1016/j.nlm.2009.05.001. Epub 2009 May 10. Neurobiol Learn Mem. 2009. PMID: 19435612 Free PMC article.
-
Hippocampal synaptic plasticity in Alzheimer's disease: what have we learned so far from transgenic models?Rev Neurosci. 2011;22(4):373-402. doi: 10.1515/RNS.2011.035. Epub 2011 Jul 6. Rev Neurosci. 2011. PMID: 21732714 Review.
-
Behaviour Hallmarks in Alzheimer's Disease 5xFAD Mouse Model.Int J Mol Sci. 2024 Jun 20;25(12):6766. doi: 10.3390/ijms25126766. Int J Mol Sci. 2024. PMID: 38928472 Free PMC article. Review.
Cited by
-
MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer's disease.Cell Mol Life Sci. 2016 Jan;73(1):217-36. doi: 10.1007/s00018-015-1992-1. Epub 2015 Jul 23. Cell Mol Life Sci. 2016. PMID: 26202697 Free PMC article.
-
Combination strategy employing BACE1 inhibitor and memantine to boost cognitive benefits in Alzheimer's disease therapy.Psychopharmacology (Berl). 2024 May;241(5):975-986. doi: 10.1007/s00213-024-06525-9. Epub 2024 Jan 10. Psychopharmacology (Berl). 2024. PMID: 38197930
-
5XFAD mice show early-onset gap encoding deficits in the auditory cortex.Neurobiol Aging. 2020 Oct;94:101-110. doi: 10.1016/j.neurobiolaging.2020.05.013. Epub 2020 Jun 1. Neurobiol Aging. 2020. PMID: 32599514 Free PMC article.
-
Spines, plasticity, and cognition in Alzheimer's model mice.Neural Plast. 2012;2012:319836. doi: 10.1155/2012/319836. Epub 2011 Nov 28. Neural Plast. 2012. PMID: 22203915 Free PMC article. Review.
-
Emerging Electroencephalographic Biomarkers to Improve Preclinical to Clinical Translation in Alzheimer's Disease.Front Aging Neurosci. 2022 Feb 17;14:805063. doi: 10.3389/fnagi.2022.805063. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35250541 Free PMC article. Review.
References
-
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. - PubMed
-
- Ashe KH. Learning and memory in transgenic mice modeling Alzheimer’s disease. Learn Mem. 2001;8:301–308. - PubMed
-
- Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, Nixon RA. Cortical plasticity in Alzheimer’s disease in humans and rodents. Biol Psychiatry. 2007;62:1405–1412. - PubMed
-
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

