Nitrotyrosinylation, remodeling and endothelial-myocyte uncoupling in iNOS, cystathionine beta synthase (CBS) knockouts and iNOS/CBS double knockout mice
- PMID: 19021146
- PMCID: PMC2668975
- DOI: 10.1002/jcb.21982
Nitrotyrosinylation, remodeling and endothelial-myocyte uncoupling in iNOS, cystathionine beta synthase (CBS) knockouts and iNOS/CBS double knockout mice
Abstract
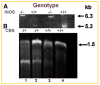
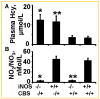
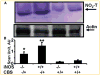
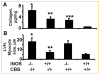
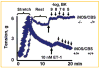
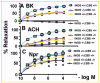
Increased levels of homocysteine (Hcy), recognized as hyperhomocysteinemia (HHcy), were associated with cardiovascular diseases. There was controversy regarding the detrimental versus cardio protective role of inducible nitric oxide synthase (iNOS) in ischemic heart disease. The aim of this study was to test the hypothesis that the Hcy generated nitrotyrosine by inducing the endothelial nitric oxide synthase, causing endothelial-myocyte (E-M) coupling. To differentiate the role of iNOS versus constitutive nitric oxide synthase (eNOS and nNOS) in Hcy-mediated nitrotyrosine generation and matrix remodeling in cardiac dysfunction, left ventricular (LV) tissue was analyzed from cystathionine beta synthase (CBS) heterozygote knockout, iNOS homozygote knockout, CBS-/+/iNOS-/- double knockout, and wild-type (WT) mice. The levels of nitrotyrosine, MMP-2 and -9 (zymographic analysis), and fibrosis (by trichrome stain) were measured. The endothelial-myocyte function was determined in cardiac rings. In CBS-/+ mice, homocysteine was elevated and in iNOS-/- mice, nitric oxide was significantly reduced. The nitrotyrosine and matrix metalloproteinase-9 (MMP-9) levels were elevated in double knockout and CBS-/+ as compared to WT mice. Although MMP-2 levels were similar in CBS-/+, iNOS-/-, and CBS-/+/iNOS-/-, the levels were three- to fourfold higher than WT. The levels of collagen were similar in CBS-/+ and iNOS-/-, but they were threefold higher than WT. Interesting, the levels of collagen increased sixfold in double knockouts, compared to WT, suggesting synergism between high Hcy and lack of iNOS. Left ventricular hypertrophy was exaggerated in the iNOS-/- and double knockout, and mildly increased in the CBS-/+, compared to WT mice. The endothelial-dependent relaxation was attenuated to the same extent in the CBS-/+ and iNOS-/-, compared to WT, but it was robustly blunted in double knockouts. The results concluded that homocysteine generated nitrotyrosine in the vicinity of endothelium, caused MMP activation and endothelium-myocyte uncoupling. The generation of nitrotyrosine was independent of iNOS.
2008 Wiley-Liss, Inc.
Figures



Similar articles
-
Functional consequences of the collagen/elastin switch in vascular remodeling in hyperhomocysteinemic wild-type, eNOS-/-, and iNOS-/- mice.Am J Physiol Lung Cell Mol Physiol. 2010 Sep;299(3):L301-11. doi: 10.1152/ajplung.00065.2010. Epub 2010 Jun 25. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20581102 Free PMC article.
-
Mesenteric vascular remodeling in hyperhomocysteinemia.Mol Cell Biochem. 2011 Feb;348(1-2):99-108. doi: 10.1007/s11010-010-0643-y. Epub 2010 Nov 13. Mol Cell Biochem. 2011. PMID: 21076854
-
3-Deazaadenosine mitigates arterial remodeling and hypertension in hyperhomocysteinemic mice.Am J Physiol Lung Cell Mol Physiol. 2006 Nov;291(5):L905-11. doi: 10.1152/ajplung.00543.2005. Epub 2006 Jun 30. Am J Physiol Lung Cell Mol Physiol. 2006. PMID: 16815886 Free PMC article.
-
Arrhythmia and neuronal/endothelial myocyte uncoupling in hyperhomocysteinemia.Arch Physiol Biochem. 2006 Oct-Dec;112(4-5):219-27. doi: 10.1080/13813450601093443. Arch Physiol Biochem. 2006. PMID: 17178594 Free PMC article. Review.
-
Mechanisms of cardiovascular remodeling in hyperhomocysteinemia.Antioxid Redox Signal. 2011 Oct 1;15(7):1927-43. doi: 10.1089/ars.2010.3721. Epub 2011 Apr 21. Antioxid Redox Signal. 2011. PMID: 21126196 Free PMC article. Review.
Cited by
-
Restoration of skeletal muscle homeostasis by hydrogen sulfide during hyperhomocysteinemia-mediated oxidative/ER stress condition 1.Can J Physiol Pharmacol. 2019 Jun;97(6):441-456. doi: 10.1139/cjpp-2018-0501. Epub 2018 Nov 13. Can J Physiol Pharmacol. 2019. PMID: 30422673 Free PMC article. Review.
-
Deficiency of iNOS Does Not Prevent Isoproterenol-induced Cardiac Hypertrophy in Mice.Korean J Physiol Pharmacol. 2009 Jun;13(3):153-9. doi: 10.4196/kjpp.2009.13.3.153. Epub 2009 Jun 30. Korean J Physiol Pharmacol. 2009. PMID: 19885031 Free PMC article.
-
Matrix imbalance by inducing expression of metalloproteinase and oxidative stress in cochlea of hyperhomocysteinemic mice.Mol Cell Biochem. 2009 Dec;332(1-2):215-24. doi: 10.1007/s11010-009-0194-2. Epub 2009 Jul 10. Mol Cell Biochem. 2009. PMID: 19590937 Free PMC article.
-
Homocysteine-mediated thrombosis and angiostasis in vascular pathobiology.J Clin Invest. 2009 Nov;119(11):3203-5. doi: 10.1172/JCI40924. Epub 2009 Oct 19. J Clin Invest. 2009. PMID: 19841539 Free PMC article.
-
Functional consequences of the collagen/elastin switch in vascular remodeling in hyperhomocysteinemic wild-type, eNOS-/-, and iNOS-/- mice.Am J Physiol Lung Cell Mol Physiol. 2010 Sep;299(3):L301-11. doi: 10.1152/ajplung.00065.2010. Epub 2010 Jun 25. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20581102 Free PMC article.
References
-
- Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R. Anti-angiogenic clues from vascular basement membrane collagen. Cancer Res. 2000;6:2520–2526. - PubMed
-
- Cox M, Sood H, Hunt M, Chandler D, Henegar J, Aru G, Tyagi SC. Apoptosis in the left ventricle of chronic volume overload causes endocardial endothelial dysfunction in rats. Am J Physiol. 2002;282:H1197–H1206. - PubMed
-
- Gattuso A, Mazza R, Pellegrino D, Tota B. Endocardial endothelium (EE) mediates luminal acetylcholine-nitric oxide signaling in isolated frog heart. Am J Physiol. 1999;276:H633–H641. - PubMed
-
- Harker LA, Slichter SJ, Scott CR, Ross R. Homocysteinemia, vascular injury and arterial thrombosis. N Engl J Med. 1974;291:537–543. - PubMed
-
- Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, Barbaro NM, Higashida RT, Dowd CF, Halbach VV, Young WL. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34:925–931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL074185/HL/NHLBI NIH HHS/United States
- R01 HL080394-03/HL/NHLBI NIH HHS/United States
- R01 HL080394/HL/NHLBI NIH HHS/United States
- R01 HL074185-05/HL/NHLBI NIH HHS/United States
- R01 HL071010/HL/NHLBI NIH HHS/United States
- R01 NS051568/NS/NINDS NIH HHS/United States
- R01 HL088012/HL/NHLBI NIH HHS/United States
- HL-74185/HL/NHLBI NIH HHS/United States
- R01 HL071010-06/HL/NHLBI NIH HHS/United States
- R01 NS051568-02/NS/NINDS NIH HHS/United States
- HL-88012/HL/NHLBI NIH HHS/United States
- HL-71010/HL/NHLBI NIH HHS/United States
- NS-51568/NS/NINDS NIH HHS/United States
- R01 HL088012-02/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

