Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease
- PMID: 19011085
- PMCID: PMC2587621
- DOI: 10.1073/pnas.0804373105
Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease
Abstract
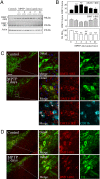
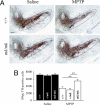
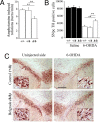
Dopaminergic cell death in the substantia nigra (SN) is central to Parkinson's disease (PD), but the neurodegenerative mechanisms have not been completely elucidated. Iron accumulation in dopaminergic and glial cells in the SN of PD patients may contribute to the generation of oxidative stress, protein aggregation, and neuronal death. The mechanisms involved in iron accumulation also remain unclear. Here, we describe an increase in the expression of an isoform of the divalent metal transporter 1 (DMT1/Nramp2/Slc11a2) in the SN of PD patients. Using the PD animal model of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication in mice, we showed that DMT1 expression increases in the ventral mesencephalon of intoxicated animals, concomitant with iron accumulation, oxidative stress, and dopaminergic cell loss. In addition, we report that a mutation in DMT1 that impairs iron transport protects rodents against parkinsonism-inducing neurotoxins MPTP and 6-hydroxydopamine. This study supports a critical role for DMT1 in iron-mediated neurodegeneration in PD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Iron transport in Parkinson's disease.Parkinsonism Relat Disord. 2009 Dec;15 Suppl 3:S209-11. doi: 10.1016/S1353-8020(09)70816-8. Parkinsonism Relat Disord. 2009. PMID: 20082992
-
Ndfip1 attenuated 6-OHDA-induced iron accumulation via regulating the degradation of DMT1.Neurobiol Aging. 2015 Feb;36(2):1183-93. doi: 10.1016/j.neurobiolaging.2014.10.021. Epub 2014 Oct 18. Neurobiol Aging. 2015. PMID: 25467637
-
S-Nitrosylation of Divalent Metal Transporter 1 Enhances Iron Uptake to Mediate Loss of Dopaminergic Neurons and Motoric Deficit.J Neurosci. 2018 Sep 26;38(39):8364-8377. doi: 10.1523/JNEUROSCI.3262-17.2018. Epub 2018 Aug 13. J Neurosci. 2018. PMID: 30104344 Free PMC article.
-
What have we learnt from CDNA microarray gene expression studies about the role of iron in MPTP induced neurodegeneration and Parkinson's disease?J Neural Transm Suppl. 2003;(65):73-88. doi: 10.1007/978-3-7091-0643-3_5. J Neural Transm Suppl. 2003. PMID: 12946050 Review.
-
The possible role of iron in the etiopathology of Parkinson's disease.Mov Disord. 1993;8(1):1-12. doi: 10.1002/mds.870080102. Mov Disord. 1993. PMID: 8419792 Review.
Cited by
-
Manganese-induced Neurotoxicity: From C. elegans to Humans.Toxicol Res (Camb). 2015 Mar 1;4(2):191-202. doi: 10.1039/C4TX00127C. Toxicol Res (Camb). 2015. PMID: 25893090 Free PMC article.
-
Iron-mediated redox modulation in neural plasticity.Commun Integr Biol. 2012 Mar 1;5(2):166-8. doi: 10.4161/cib.18710. Commun Integr Biol. 2012. PMID: 22808323 Free PMC article.
-
Lysosomal dysfunction in α-synuclein pathology: molecular mechanisms and therapeutic strategies.Cell Mol Life Sci. 2024 Sep 3;81(1):382. doi: 10.1007/s00018-024-05419-5. Cell Mol Life Sci. 2024. PMID: 39223418 Free PMC article. Review.
-
TMEM106B Knockdown Exhibits a Neuroprotective Effect in Parkinson's Disease via Decreasing Inflammation and Iron Deposition.Mol Neurobiol. 2025 Feb;62(2):1813-1825. doi: 10.1007/s12035-024-04373-4. Epub 2024 Jul 23. Mol Neurobiol. 2025. PMID: 39044012 Free PMC article.
-
Ginsenoside Rg1 Plays a Neuroprotective Role in Regulating the Iron-Regulated Proteins and Against Lipid Peroxidation in Oligodendrocytes.Neurochem Res. 2022 Jun;47(6):1721-1735. doi: 10.1007/s11064-022-03564-6. Epub 2022 Feb 28. Neurochem Res. 2022. PMID: 35229270
References
-
- Forno LS. Neuropathology of Parkinson's disease. Neuropathol Exp Neurol. 1996;55:259–272. - PubMed
-
- Sofic E, et al. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 1988;74:199–205. - PubMed
-
- Hirsch EC, Brandel JP, Galle P, Javoy-Agid F, Agid Y. Iron and aluminum increase in the substantia nigra of patients with Parkinson's disease: An X-ray microanalysis. J Neurochem. 1991;56:446–451. - PubMed
-
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular link between Parkinson's disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. - PubMed
-
- Kaur D, et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: A novel therapy for Parkinson's disease. Neuron. 2003;37:899–909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

