G-protein-coupled receptor screen reveals a role for chemokine receptor CCR5 in suppressing microglial neurotoxicity
- PMID: 19005063
- PMCID: PMC6671655
- DOI: 10.1523/JNEUROSCI.2920-08.2008
G-protein-coupled receptor screen reveals a role for chemokine receptor CCR5 in suppressing microglial neurotoxicity
Abstract
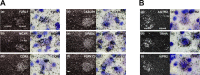
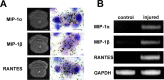
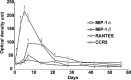
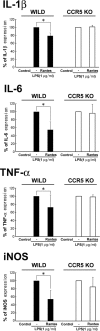
G-protein-coupled receptors (GPCRs) form the largest superfamily of membrane proteins, and several GPCRs have been implicated in signaling between neurons and glia to protect neurons from pathological stresses. Here, we have used a screening strategy to investigate GPCRs that are involved in neuronal protection. The real-time PCR was performed using 274 primers targeting nonsensory GPCR mRNAs, which were listed on the database. The cDNAs from control and nerve-injured hypoglossal nuclei of mouse brain were used, and the alterations of PCR products were compared. This screen and the subsequent in situ hybridization screen exhibited six GPCR mRNAs which were prominently and convincingly induced in nerve-injured hypoglossal nuclei. Among these candidates, the chemokine receptor CCR5 was selected, based on the marked induction in CCR5 mRNA in microglia after nerve injury. The mRNA expression of ligands for CCR5, such as regulated on activation normal T-cell expressed and secreted (RANTES/CCL5), MIP-1alpha, and MIP-1beta, were induced in injured motor neurons, indicating that CCR5 and its ligands were expressed in microglia and neurons, respectively, in response to nerve injury. In vitro, lipopolysaccharide (LPS)-induced expression of mRNAs for inflammatory cytokines (IL-1beta, IL-6, and tumor necrosis factor-alpha) and inducible nitric oxide synthase (iNOS) in microglia were all suppressed by RANTES. Those suppressions were not observed in microglia from CCR5 null mice. In addition, nerve injury-induced motor neuron death seen in wild type C56BL/6J mice was accelerated in CCR5 knock-out C57BL/6J. These results may suggest that CCR5-mediated neuron-glia signaling functions to protect neurons by suppressing microglia toxicity.
Figures
Similar articles
-
MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4.J Leukoc Biol. 2005 Jul;78(1):167-77. doi: 10.1189/jlb.0404237. Epub 2005 Apr 14. J Leukoc Biol. 2005. PMID: 15831559
-
CCR5 chemokine receptor mediates recruitment of MHC class II-positive Langerhans cells in the mouse corneal epithelium.Invest Ophthalmol Vis Sci. 2005 Apr;46(4):1201-7. doi: 10.1167/iovs.04-0658. Invest Ophthalmol Vis Sci. 2005. PMID: 15790880
-
Role of interleukin-4, the chemokine CCL3 and its receptor CCR5 in neuropathic pain.Mol Immunol. 2016 Sep;77:184-92. doi: 10.1016/j.molimm.2016.08.006. Epub 2016 Aug 11. Mol Immunol. 2016. PMID: 27522478
-
Relationship between the chemokine receptor CCR5 and microglia in neurological disorders: consequences of targeting CCR5 on neuroinflammation, neuronal death and regeneration in a model of epilepsy.CNS Neurol Disord Drug Targets. 2013 Sep;12(6):815-29. doi: 10.2174/18715273113126660173. CNS Neurol Disord Drug Targets. 2013. PMID: 24047524 Review.
-
The intricate role of CCL5/CCR5 axis in Alzheimer disease.J Neuropathol Exp Neurol. 2023 Oct 20;82(11):894-900. doi: 10.1093/jnen/nlad071. J Neuropathol Exp Neurol. 2023. PMID: 37769321 Free PMC article. Review.
Cited by
-
Mitochondrial fission is an acute and adaptive response in injured motor neurons.Sci Rep. 2016 Jun 20;6:28331. doi: 10.1038/srep28331. Sci Rep. 2016. PMID: 27319806 Free PMC article.
-
Transcriptome analysis of the effect of C-C chemokine receptor 5 deficiency on cell response to Toxoplasma gondii in brain cells.BMC Genomics. 2019 Sep 11;20(1):705. doi: 10.1186/s12864-019-6076-4. BMC Genomics. 2019. PMID: 31506064 Free PMC article.
-
Recombinant tissue plasminogen activator enhances microglial cell recruitment after stroke in mice.J Cereb Blood Flow Metab. 2014 May;34(5):802-12. doi: 10.1038/jcbfm.2014.9. Epub 2014 Jan 29. J Cereb Blood Flow Metab. 2014. PMID: 24473480 Free PMC article.
-
The enhancement of CCL2 and CCL5 by human bone marrow-derived mesenchymal stem/stromal cells might contribute to inflammatory suppression and axonal extension after spinal cord injury.PLoS One. 2020 Mar 10;15(3):e0230080. doi: 10.1371/journal.pone.0230080. eCollection 2020. PLoS One. 2020. PMID: 32155215 Free PMC article.
-
Identifying methamphetamine use predictors in HIV infection: Immune-dopaminergic signatures in peripheral leukocytes and the role of COMT genotype.Brain Behav Immun Health. 2024 Oct 5;42:100873. doi: 10.1016/j.bbih.2024.100873. eCollection 2024 Dec. Brain Behav Immun Health. 2024. PMID: 39430881 Free PMC article.
References
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. - PubMed
-
- Balistreri CR, Caruso C, Grimaldi MP, Listì F, Vasto S, Orlando V, Campagna AM, Lio D, Candore G. CCR5 receptor: biologic and genetic implications in age-related diseases. Ann NY Acad Sci. 2007;1100:162–172. - PubMed
-
- Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
