Activation of beta-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration
- PMID: 18991257
- PMCID: PMC4437562
- DOI: 10.1002/pros.20877
Activation of beta-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration
Abstract
Background: The role of Wnt/beta-Catenin signaling in embryogenesis and carcinogenesis has been extensively studied in organs such as colon, lung and pancreas, but little is known about Wnt/beta-Catenin signaling in the prostate. Although stabilizing mutations in APC and beta-Catenin are rare in primary prostate tumors, recent studies suggest that cytoplasmic/nuclear beta-Catenin is associated with advanced, metastatic, hormone-refractory prostate carcinoma.
Methods: To better understand the role of beta-Catenin in prostatic development and carcinogenesis, we studied Wnt expression during prostate development and activated Wnt/beta-Catenin signaling in the developing and adult prostate.
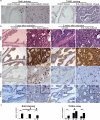
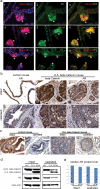
Results: Our results demonstrated that during prostate development Wnt ligands display a dynamic expression pattern. Activation of beta-Catenin during prostate development caused epithelial hyperplasia followed by prostatic intraepithelial neoplasia (PIN) in prostate. In the adult prostate, activation of beta-Catenin resulted in high grade PIN (HGPIN) and continuous prostatic growth after castration. As a result of activation of beta-Catenin, AR was first up-regulated with the emergence of epithelial hyperplasia, but was later down-regulated when HGPIN developed. Furthermore, activation of beta-Catenin induced Foxa2 re-expression in adult prostate which normally is only expressed in the embryonic budding stage during prostate development.
Conclusions: The results from this study strongly suggest that Wnt/beta-Catenin signaling is involved in the regulation of prostate development and confirm that constitutive activation of this pathway enables the mouse prostate to grow after castration.
Figures




Similar articles
-
Activation of Wnt/β-catenin signaling in a subpopulation of murine prostate luminal epithelial cells induces high grade prostate intraepithelial neoplasia.Prostate. 2014 Nov;74(15):1506-20. doi: 10.1002/pros.22868. Epub 2014 Aug 29. Prostate. 2014. PMID: 25175604 Free PMC article.
-
Androgen signaling is a confounding factor for β-catenin-mediated prostate tumorigenesis.Oncogene. 2016 Feb 11;35(6):702-14. doi: 10.1038/onc.2015.117. Epub 2015 Apr 20. Oncogene. 2016. PMID: 25893287 Free PMC article.
-
Inactivation of Apc in the mouse prostate causes prostate carcinoma.Cancer Res. 2007 Mar 15;67(6):2490-6. doi: 10.1158/0008-5472.CAN-06-3028. Cancer Res. 2007. PMID: 17363566
-
Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer.J Cell Biochem. 2006 Oct 1;99(2):402-10. doi: 10.1002/jcb.20983. J Cell Biochem. 2006. PMID: 16741972 Review.
-
Roles and regulation of Wnt signaling and beta-catenin in prostate cancer.Cancer Lett. 2006 Jun 8;237(1):22-32. doi: 10.1016/j.canlet.2005.06.004. Epub 2005 Jul 14. Cancer Lett. 2006. PMID: 16023783 Review.
Cited by
-
Wnt/β-catenin signalling in prostate cancer.Nat Rev Urol. 2012 Aug;9(8):418-28. doi: 10.1038/nrurol.2012.116. Epub 2012 Jun 19. Nat Rev Urol. 2012. PMID: 22710668 Review.
-
Molecular genetics of prostate cancer: new prospects for old challenges.Genes Dev. 2010 Sep 15;24(18):1967-2000. doi: 10.1101/gad.1965810. Genes Dev. 2010. PMID: 20844012 Free PMC article. Review.
-
Interplay Between SOX9, Wnt/β-Catenin and Androgen Receptor Signaling in Castration-Resistant Prostate Cancer.Int J Mol Sci. 2019 Apr 26;20(9):2066. doi: 10.3390/ijms20092066. Int J Mol Sci. 2019. PMID: 31027362 Free PMC article. Review.
-
Differentiation of the ductal epithelium and smooth muscle in the prostate gland are regulated by the Notch/PTEN-dependent mechanism.Dev Biol. 2011 Aug 15;356(2):337-49. doi: 10.1016/j.ydbio.2011.05.659. Epub 2011 May 20. Dev Biol. 2011. PMID: 21624358 Free PMC article.
-
The many faces of neuroendocrine differentiation in prostate cancer progression.Front Oncol. 2014 Mar 25;4:60. doi: 10.3389/fonc.2014.00060. eCollection 2014. Front Oncol. 2014. PMID: 24724054 Free PMC article. Review.
References
-
- Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26:898–915. - PubMed
-
- Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC. The androgen receptor can promote Beta -catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem. 2002;277:17933–43. - PubMed
-
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA059705/CA/NCI NIH HHS/United States
- R01 AG023490-04/AG/NIA NIH HHS/United States
- R01 CA115985/CA/NCI NIH HHS/United States
- R01-CA115985/CA/NCI NIH HHS/United States
- R01 DK076602/DK/NIDDK NIH HHS/United States
- R01-CA113392/CA/NCI NIH HHS/United States
- R01 CA076142/CA/NCI NIH HHS/United States
- R01 AG023490/AG/NIA NIH HHS/United States
- R01-CA59705/CA/NCI NIH HHS/United States
- R01-DK55748/DK/NIDDK NIH HHS/United States
- R01 DK055748/DK/NIDDK NIH HHS/United States
- R01 CA113392/CA/NCI NIH HHS/United States
- R01-DK076602/DK/NIDDK NIH HHS/United States
- R01-AG023490/AG/NIA NIH HHS/United States
- R01-CA76142/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

