Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates
- PMID: 18990132
- PMCID: PMC3753195
- DOI: 10.1196/annals.1444.017
Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates
Abstract
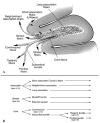
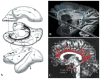
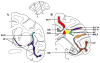
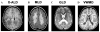
Lesions of the cerebral white matter (WM) result in focal neurobehavioral syndromes, neuropsychiatric phenomena, and dementia. The cerebral WM contains fiber pathways that convey axons linking cerebral cortical areas with each other and with subcortical structures, facilitating the distributed neural circuits that subserve sensorimotor function, intellect, and emotion. Recent neuroanatomical investigations reveal that these neural circuits are topographically linked by five groupings of fiber tracts emanating from every neocortical area: (1) cortico-cortical association fibers; (2) corticostriatal fibers; (3) commissural fibers; and cortico-subcortical pathways to (4) thalamus and (5) pontocerebellar system, brain stem, and/or spinal cord. Lesions of association fibers prevent communication between cortical areas engaged in different domains of behavior. Lesions of subcortical structures or projection/striatal fibers disrupt the contribution of subcortical nodes to behavior. Disconnection syndromes thus result from lesions of the cerebral cortex, subcortical structures, and WM tracts that link the nodes that make up the distributed circuits. The nature and the severity of the clinical manifestations of WM lesions are determined, in large part, by the location of the pathology: discrete neurological and neuropsychiatric symptoms result from focal WM lesions, whereas cognitive impairment across multiple domains--WM dementia--occurs in the setting of diffuse WM disease. We present a detailed review of the conditions affecting WM that produce these neurobehavioral syndromes, and consider the pathophysiology, clinical effects, and broad significance of the effects of aging and vascular compromise on cerebral WM, in an attempt to help further the understanding, diagnosis, and treatment of these disorders.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

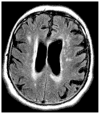

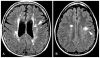
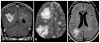
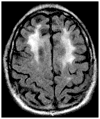

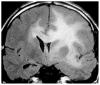
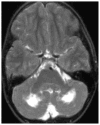
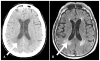
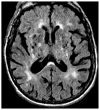
Similar articles
-
Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems.Cortex. 2008 Sep;44(8):1037-66. doi: 10.1016/j.cortex.2008.04.004. Epub 2008 May 23. Cortex. 2008. PMID: 18614161 Free PMC article.
-
White matter and behavioral neurology.Ann N Y Acad Sci. 2005 Dec;1064:162-83. doi: 10.1196/annals.1340.028. Ann N Y Acad Sci. 2005. PMID: 16394155 Review.
-
Diffusion tensor imaging in presymptomatic and early Huntington's disease: Selective white matter pathology and its relationship to clinical measures.Mov Disord. 2006 Sep;21(9):1317-25. doi: 10.1002/mds.20979. Mov Disord. 2006. PMID: 16755582
-
Contribution of cortical and white matter lesions to cognitive impairment in multiple sclerosis.Mult Scler. 2013 Sep;19(10):1290-6. doi: 10.1177/1352458513475490. Epub 2013 Mar 4. Mult Scler. 2013. PMID: 23459568
-
The behavioral neurology of cerebral white matter.Neurology. 1998 Jun;50(6):1535-40. doi: 10.1212/wnl.50.6.1535. Neurology. 1998. PMID: 9633691 Review.
Cited by
-
Sleep and Cognitive Abnormalities in Acute Minor Thalamic Infarction.Neurosci Bull. 2016 Aug;32(4):341-8. doi: 10.1007/s12264-016-0036-7. Epub 2016 May 30. Neurosci Bull. 2016. PMID: 27237578 Free PMC article.
-
White Matter Correlates of Theory of Mind in Patients With First-Episode Psychosis.Front Psychiatry. 2021 Mar 5;12:617683. doi: 10.3389/fpsyt.2021.617683. eCollection 2021. Front Psychiatry. 2021. PMID: 33746794 Free PMC article.
-
Prospective assessment of white matter integrity in adult stem cell transplant recipients.Brain Imaging Behav. 2016 Jun;10(2):486-96. doi: 10.1007/s11682-015-9423-3. Brain Imaging Behav. 2016. PMID: 26153467 Free PMC article.
-
The impact of levamisole and alcohol on white matter microstructure in adult chronic cocaine users.Addict Biol. 2022 May;27(3):e13149. doi: 10.1111/adb.13149. Addict Biol. 2022. PMID: 35394690 Free PMC article.
-
The organization of local and distant functional connectivity in the human brain.PLoS Comput Biol. 2010 Jun 10;6(6):e1000808. doi: 10.1371/journal.pcbi.1000808. PLoS Comput Biol. 2010. PMID: 20548945 Free PMC article.
References
-
- Neuburger M. Die historische Entwicklung der experimentellen Gehirn- und Rückenmarksphysiologie vor Flourens. Ferdinand Enke Verlag, Stuttgart. Translated and edited, with additional material, by Edwin Clarke (1981) The Historical Development of Experimental Brain and Spinal Cord Physiology before Flourens. Johns Hopkins University Press; Baltimore and London: 1897.
-
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press; New York: 2006.
-
- Schmahmann JD, Pandya DN. Cerebral white matter–historical evolution of facts and notions concerning the organization of the fiber pathways of the brain. J Hist Neurosci. 2007;16:237–267. - PubMed
-
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. - PubMed
-
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585–644. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

