Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7
- PMID: 18988920
- PMCID: PMC2689916
- DOI: 10.1165/rcmb.2008-0217OC
Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7
Abstract
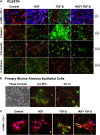
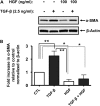
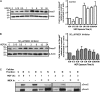
Idiopathic pulmonary fibrosis is a lethal parenchymal lung disease characterized by denudation of the lung epithelium, fibroblast proliferation, and collagen deposition. Cellular changes underlying disease progression involve injury to alveolar epithelial cells, epithelial to mesenchymal transition, proliferation of alpha-smooth muscle actin (alpha-SMA)-expressing myofibroblasts and of fibroblasts resulting in enhanced deposition of extracellular matrix proteins. Hepatocyte growth factor (HGF) inhibits progression of bleomycin-induced pulmonary fibrosis in mice. The mechanism underlying the inhibitory effect of HGF was investigated in an in vitro model. We show that HGF markedly antagonizes basal and transforming growth factor (TGF)-beta-induced expression of myofibroblast markers such as alpha-SMA, collagen type 1, and fibronectin in rat alveolar epithelial cells. HGF also inhibited TGF-beta-induced alpha-SMA expression in primary murine alveolar epithelial cells. Since TGF-beta is known to regulate alpha-SMA expression, the effect of HGF on components of TGF-beta signaling was investigated. HGF induced expression of Smad7, an inhibitor of TGF-beta signaling, in a mitogen-activated protein kinase-dependent manner. HGF also induced the nuclear export of Smad7 and Smad ubiquitin regulatory factor 1 (Smurf1) to the cytoplasm. HGF-dependent decrease in alpha-SMA was abolished with specific siRNAs targeted to Smad7. Thus, induction of Smad7 by HGF serves to limit acquisition of the myofibroblast phenotype in alveolar epithelial cells.
Figures


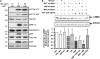

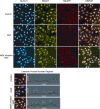
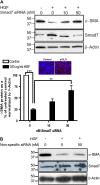
Similar articles
-
Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction.Am J Pathol. 2003 Aug;163(2):621-32. doi: 10.1016/S0002-9440(10)63689-9. Am J Pathol. 2003. PMID: 12875981 Free PMC article.
-
Smad7 blocks transforming growth factor-β1-induced gingival fibroblast-myofibroblast transition via inhibitory regulation of Smad2 and connective tissue growth factor.J Periodontol. 2011 Apr;82(4):642-51. doi: 10.1902/jop.2010.100510. Epub 2010 Nov 8. J Periodontol. 2011. PMID: 21054221
-
[Tubular epithelial-myofibroblast transdifferentiation and expressions of hepatocyte growth factor and Smad7 in renal tissues of rat with experimental diabetes].Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005 Nov;17(11):675-8. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005. PMID: 16297324 Chinese.
-
Hepatocyte growth factor in lung repair and pulmonary fibrosis.Acta Pharmacol Sin. 2011 Jan;32(1):12-20. doi: 10.1038/aps.2010.90. Epub 2010 Dec 6. Acta Pharmacol Sin. 2011. PMID: 21131996 Free PMC article. Review.
-
Reciprocal functions of hepatocyte growth factor and transforming growth factor-beta1 in the progression of renal diseases: a role for CD44?Kidney Int Suppl. 2003 Oct;(86):S15-20. doi: 10.1046/j.1523-1755.64.s86.4.x. Kidney Int Suppl. 2003. PMID: 12969122 Review.
Cited by
-
Enzyme-like nanoparticle-engineered mesenchymal stem cell secreting HGF promotes visualized therapy for idiopathic pulmonary fibrosis in vivo.Sci Adv. 2024 Aug 23;10(34):eadq0703. doi: 10.1126/sciadv.adq0703. Epub 2024 Aug 21. Sci Adv. 2024. PMID: 39167646 Free PMC article.
-
Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway.Stem Cell Res Ther. 2016 Aug 2;7(1):102. doi: 10.1186/s13287-016-0356-6. Stem Cell Res Ther. 2016. PMID: 27484727 Free PMC article.
-
RhoA-Dependent HGF and c-Met Mediate Gas6-Induced Inhibition of Epithelial-Mesenchymal Transition, Migration, and Invasion of Lung Alveolar Epithelial Cells.Biomolecules. 2019 Oct 4;9(10):565. doi: 10.3390/biom9100565. Biomolecules. 2019. PMID: 31590238 Free PMC article.
-
miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis.J Exp Med. 2010 Aug 2;207(8):1589-97. doi: 10.1084/jem.20100035. Epub 2010 Jul 19. J Exp Med. 2010. PMID: 20643828 Free PMC article.
-
Regulation of hepatocyte growth factor in mice with pneumonia by peptidases and trans-alveolar flux.PLoS One. 2015 May 4;10(5):e0125797. doi: 10.1371/journal.pone.0125797. eCollection 2015. PLoS One. 2015. PMID: 25938594 Free PMC article.
References
-
- Selman M. Idiopathic pulmonary fibrosis challenges for the future. Chest 2001;120:8–10. - PubMed
-
- Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 2006;3:364–372. - PubMed
-
- Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004;55:395–417. - PubMed
-
- Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res 2007;24:819–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

