Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection
- PMID: 18987148
- PMCID: PMC2612355
- DOI: 10.1128/JVI.02036-08
Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection
Erratum in
- J Virol. 2009 May;83(9):4713-5
Abstract
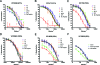
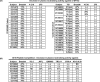
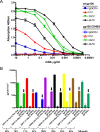
The characterization of the cross-reactive, or heterologous, neutralizing antibody responses developed during human immunodeficiency virus type 1 (HIV-1) infection and the identification of factors associated with their generation are relevant to the development of an HIV vaccine. We report that in healthy HIV-positive, antiretroviral-naïve subjects, the breadth of plasma heterologous neutralizing antibody responses correlates with the time since infection, plasma viremia levels, and the binding avidity of anti-Env antibodies. Anti-CD4-binding site antibodies are responsible for the exceptionally broad cross-neutralizing antibody responses recorded only in rare plasma samples. However, in most cases examined, antibodies to the variable regions and to the CD4-binding site of Env modestly contributed in defining the overall breadth of these responses. Plasmas with broad cross-neutralizing antibody responses were identified that targeted the gp120 subunit, but their precise epitopes mapped outside the variable regions and the CD4-binding site. Finally, although several plasmas were identified with cross-neutralizing antibody responses that were not directed against gp120, only one plasma with a moderate breadth of heterologous neutralizing antibody responses contained cross-reactive neutralizing antibodies against the 4E10 epitope, which is within the gp41 transmembrane subunit. Overall, our study indicates that more than one pathway leads to the development of broad cross-reactive neutralizing antibodies during HIV infection and that the virus continuously escapes their action.
Figures




Similar articles
-
Epitope specificities of broadly neutralizing plasmas from HIV-1 infected subjects.Vaccine. 2010 May 26;28 Suppl 2:B8-12. doi: 10.1016/j.vaccine.2009.07.085. Vaccine. 2010. PMID: 20510750 Free PMC article.
-
Evolution of cross-neutralizing antibody specificities to the CD4-BS and the carbohydrate cloak of the HIV Env in an HIV-1-infected subject.PLoS One. 2012;7(11):e49610. doi: 10.1371/journal.pone.0049610. Epub 2012 Nov 13. PLoS One. 2012. PMID: 23152926 Free PMC article.
-
Increased breadth of HIV-1 neutralization achieved by diverse antibody clones each with limited neutralization breadth.PLoS One. 2018 Dec 19;13(12):e0209437. doi: 10.1371/journal.pone.0209437. eCollection 2018. PLoS One. 2018. PMID: 30566528 Free PMC article.
-
Conformational Masking and Receptor-Dependent Unmasking of Highly Conserved Env Epitopes Recognized by Non-Neutralizing Antibodies That Mediate Potent ADCC against HIV-1.Viruses. 2015 Sep 18;7(9):5115-32. doi: 10.3390/v7092856. Viruses. 2015. PMID: 26393642 Free PMC article. Review.
-
Interfer'n with antibody responses.Sci Immunol. 2016 Oct 21;1(4):eaaj1836. doi: 10.1126/sciimmunol.aaj1836. Sci Immunol. 2016. PMID: 28783688 Review.
Cited by
-
Coverage of primary mother-to-child HIV transmission isolates by second-generation broadly neutralizing antibodies.AIDS. 2013 Jan 28;27(3):337-46. doi: 10.1097/QAD.0b013e32835cadd6. AIDS. 2013. PMID: 23296195 Free PMC article.
-
Optimal sequence-based design for multi-antigen HIV-1 vaccines using minimally distant antigens.PLoS Comput Biol. 2022 Oct 31;18(10):e1010624. doi: 10.1371/journal.pcbi.1010624. eCollection 2022 Oct. PLoS Comput Biol. 2022. PMID: 36315492 Free PMC article.
-
CRF01_AE-specific neutralizing activity observed in plasma derived from HIV-1-infected Thai patients residing in northern Thailand: comparison of neutralizing breadth and potency between plasma derived from rapid and slow progressors.PLoS One. 2013;8(1):e53920. doi: 10.1371/journal.pone.0053920. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308290 Free PMC article.
-
HIV-1 superinfection in women broadens and strengthens the neutralizing antibody response.PLoS Pathog. 2012;8(3):e1002611. doi: 10.1371/journal.ppat.1002611. Epub 2012 Mar 29. PLoS Pathog. 2012. PMID: 22479183 Free PMC article.
-
Longitudinal analysis of early HIV-1-specific neutralizing activity in an elite neutralizer and in five patients who developed cross-reactive neutralizing activity.J Virol. 2012 Feb;86(4):2045-55. doi: 10.1128/JVI.06091-11. Epub 2011 Dec 7. J Virol. 2012. PMID: 22156522 Free PMC article.
References
-
- Backliwal, G., M. Hildinger, V. Hasija, and F. M. Wurm. 2008. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol. Bioeng. 99721-727. - PubMed
-
- Binley, J. M., E. A. Lybarger, E. T. Crooks, M. S. Seaman, E. Gray, K. L. Davis, J. M. Decker, D. Wycuff, L. Harris, N. Hawkins, B. Wood, C. Nathe, D. Richman, G. D. Tomaras, F. Bibollet-Ruche, J. E. Robinson, L. Morris, G. M. Shaw, D. C. Montefiori, and J. R. Mascola. 24 September 2008. Profiling the specificity of neutralizing antibodies in a large panel of HIV-1 plasmas from subtype B and C chronic infections. J. Virol. 8211651-11668. - PMC - PubMed
-
- Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. - PMC - PubMed
-
- Blish, C. A., R. Nedellec, K. Mandaliya, D. E. Mosier, and J. Overbaugh. 2007. HIV-1 subtype A envelope variants from early in infection have variable sensitivity to neutralization and to inhibitors of viral entry. AIDS 21693-702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

