Cysteine pK(a) values for the bacterial peroxiredoxin AhpC
- PMID: 18986167
- PMCID: PMC2645924
- DOI: 10.1021/bi801718d
Cysteine pK(a) values for the bacterial peroxiredoxin AhpC
Abstract
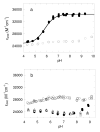
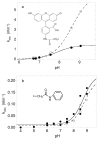
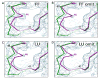
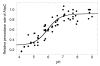
Salmonella typhimurium AhpC is a founding member of the peroxiredoxin family, a ubiquitous group of cysteine-based peroxidases with high reactivity toward hydrogen peroxide, organic hydroperoxides, and peroxynitrite. For all of the peroxiredoxins, the catalytic cysteine, referred to as the peroxidatic cysteine (C(P)), acts as a nucleophile in attacking the peroxide substrate, forming a cysteine sulfenic acid at the active site. Because thiolates are far stronger nucleophiles than thiol groups, it is generally accepted that cysteine-based peroxidases should exhibit pK(a) values lower than an unperturbed value of 8.3-8.5. In this investigation, several independent approaches were used to assess the pK(a) of the two cysteinyl residues of AhpC. Methods using two different iodoacetamide derivatives yielded unperturbed pK(a) values (7.9-8.7) for both cysteines, apparently due to reactivity with the wrong conformation of C(P) (i.e., locally unfolded and flipped out of the active site), as supported by X-ray crystallographic analyses. A functional pK(a) of 5.94 +/- 0.10 presumably reflecting the titration of C(P) within the fully folded active site was obtained by measuring AhpC competition with horseradish peroxidase for hydrogen peroxide; this value is quite similar to that obtained by analyzing the pH dependence of the epsilon(240) of wild-type AhpC (5.84 +/- 0.02) and similar to those obtained for two typical 2-cysteine peroxiredoxins from Saccharomyces cerevisiae (5.4 and 6.0). Thus, the pK(a) value of AhpC balances the need for a deprotonated thiol (at pH 7, approximately 90% of the C(P) would be deprotonated) with the fact that thiolates with higher pK(a) values are stronger nucleophiles.
Figures
Similar articles
-
Roles for the two cysteine residues of AhpC in catalysis of peroxide reduction by alkyl hydroperoxide reductase from Salmonella typhimurium.Biochemistry. 1997 Oct 28;36(43):13349-56. doi: 10.1021/bi9713658. Biochemistry. 1997. PMID: 9341227
-
Experimentally Dissecting the Origins of Peroxiredoxin Catalysis.Antioxid Redox Signal. 2018 Mar 1;28(7):521-536. doi: 10.1089/ars.2016.6922. Epub 2017 Apr 4. Antioxid Redox Signal. 2018. PMID: 28375740 Free PMC article.
-
Bacterial defenses against oxidants: mechanistic features of cysteine-based peroxidases and their flavoprotein reductases.Arch Biochem Biophys. 2005 Jan 1;433(1):240-54. doi: 10.1016/j.abb.2004.09.006. Arch Biochem Biophys. 2005. PMID: 15581580 Review.
-
Dissecting peroxiredoxin catalysis: separating binding, peroxidation, and resolution for a bacterial AhpC.Biochemistry. 2015 Feb 24;54(7):1567-75. doi: 10.1021/bi501515w. Epub 2015 Feb 10. Biochemistry. 2015. PMID: 25633283 Free PMC article.
-
Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite.Subcell Biochem. 2007;44:83-113. doi: 10.1007/978-1-4020-6051-9_5. Subcell Biochem. 2007. PMID: 18084891 Review.
Cited by
-
Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins.Methods Enzymol. 2010;473:77-94. doi: 10.1016/S0076-6879(10)73003-2. Methods Enzymol. 2010. PMID: 20513472 Free PMC article. Review.
-
A structural and mechanistic study of π-clamp-mediated cysteine perfluoroarylation.Sci Rep. 2017 Aug 11;7(1):7954. doi: 10.1038/s41598-017-08402-2. Sci Rep. 2017. PMID: 28801573 Free PMC article.
-
Piecing Together How Peroxiredoxins Maintain Genomic Stability.Antioxidants (Basel). 2018 Nov 28;7(12):177. doi: 10.3390/antiox7120177. Antioxidants (Basel). 2018. PMID: 30486489 Free PMC article. Review.
-
Unique Cellular and Biochemical Features of Human Mitochondrial Peroxiredoxin 3 Establish the Molecular Basis for Its Specific Reaction with Thiostrepton.Antioxidants (Basel). 2021 Jan 20;10(2):150. doi: 10.3390/antiox10020150. Antioxidants (Basel). 2021. PMID: 33498547 Free PMC article.
-
Peroxiredoxin-1 from the human hookworm Ancylostoma ceylanicum forms a stable oxidized decamer and is covalently inhibited by conoidin A.Chem Biol. 2013 Aug 22;20(8):991-1001. doi: 10.1016/j.chembiol.2013.06.011. Epub 2013 Jul 25. Chem Biol. 2013. PMID: 23891152 Free PMC article.
References
-
- Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. - PubMed
-
- Wood ZA, Schröder E, Harris JR, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. - PubMed
-
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

