Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells
- PMID: 18984736
- PMCID: PMC2606868
- DOI: 10.2337/db08-0862
Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells
Abstract
Objective: Calcium-permeable cation channel TRPV2 is expressed in pancreatic beta-cells. We investigated regulation and function of TRPV2 in beta-cells.
Research design and methods: Translocation of TRPV2 was assessed in MIN6 cells and cultured mouse beta-cells by transfecting TRPV2 fused to green fluorescent protein or TRPV2 containing c-Myc tag in the extracellular domain. Calcium entry was assessed by monitoring fura-2 fluorescence.
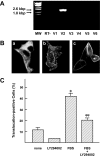
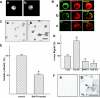
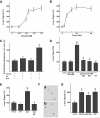
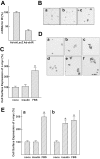
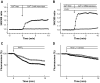
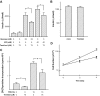
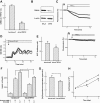
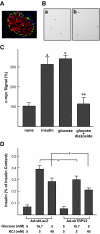
Results: In MIN6 cells, TRPV2 was observed mainly in cytoplasm in an unstimulated condition. Addition of exogenous insulin induced translocation and insertion of TRPV2 to the plasma membrane. Consistent with these observations, insulin increased calcium entry, which was inhibited by tranilast, an inhibitor of TRPV2, or by knockdown of TRPV2 using shRNA. A high concentration of glucose also induced translocation of TRPV2, which was blocked by nefedipine, diazoxide, and somatostatin, agents blocking glucose-induced insulin secretion. Knockdown of the insulin receptor attenuated insulin-induced translocation of TRPV2. Similarly, the effect of insulin on TRPV2 translocation was not observed in a beta-cell line derived from islets obtained from a beta-cell-specific insulin receptor knockout mouse. Knockdown of TRPV2 or addition of tranilast significantly inhibited insulin secretion induced by a high concentration of glucose. Likewise, cell growth induced by serum and glucose was inhibited by tranilast or by knockdown of TRPV2. Finally, insulin-induced translocation of TRPV2 was observed in cultured mouse beta-cells, and knockdown of TRPV2 reduced insulin secretion induced by glucose.
Conclusions: TRPV2 is regulated by insulin and is involved in the autocrine action of this hormone on beta-cells.
Figures
Similar articles
-
Antiaging gene Klotho enhances glucose-induced insulin secretion by up-regulating plasma membrane levels of TRPV2 in MIN6 β-cells.Endocrinology. 2012 Jul;153(7):3029-39. doi: 10.1210/en.2012-1091. Epub 2012 May 17. Endocrinology. 2012. PMID: 22597535 Free PMC article.
-
TRPV2 channels mediate insulin secretion induced by cell swelling in mouse pancreatic β-cells.Am J Physiol Cell Physiol. 2019 Mar 1;316(3):C434-C443. doi: 10.1152/ajpcell.00210.2017. Epub 2019 Jan 16. Am J Physiol Cell Physiol. 2019. PMID: 30649920
-
Insulin/phosphoinositide 3-kinase pathway accelerates the glucose-induced first-phase insulin secretion through TrpV2 recruitment in pancreatic β-cells.Biochem J. 2010 Dec 1;432(2):375-86. doi: 10.1042/BJ20100864. Biochem J. 2010. PMID: 20854263
-
TRPV2.Handb Exp Pharmacol. 2014;222:247-72. doi: 10.1007/978-3-642-54215-2_10. Handb Exp Pharmacol. 2014. PMID: 24756709 Review.
-
The role of thermosensitive TRP (transient receptor potential) channels in insulin secretion.Endocr J. 2011;58(12):1021-8. doi: 10.1507/endocrj.ej11-0130. Epub 2011 Jul 23. Endocr J. 2011. PMID: 21785227 Review.
Cited by
-
Antiaging gene Klotho enhances glucose-induced insulin secretion by up-regulating plasma membrane levels of TRPV2 in MIN6 β-cells.Endocrinology. 2012 Jul;153(7):3029-39. doi: 10.1210/en.2012-1091. Epub 2012 May 17. Endocrinology. 2012. PMID: 22597535 Free PMC article.
-
Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation.Br J Pharmacol. 2014 May;171(9):2426-39. doi: 10.1111/bph.12615. Br J Pharmacol. 2014. PMID: 24641282 Free PMC article.
-
Understanding the cellular function of TRPV2 channel through generation of specific monoclonal antibodies.PLoS One. 2013 Dec 31;8(12):e85392. doi: 10.1371/journal.pone.0085392. eCollection 2013. PLoS One. 2013. PMID: 24392006 Free PMC article.
-
Trafficking of ThermoTRP Channels.Membranes (Basel). 2014 Aug 19;4(3):525-64. doi: 10.3390/membranes4030525. Membranes (Basel). 2014. PMID: 25257900 Free PMC article. Review.
-
Cannabidiol sensitizes TRPV2 channels to activation by 2-APB.bioRxiv [Preprint]. 2023 Jan 27:2023.01.27.525817. doi: 10.1101/2023.01.27.525817. bioRxiv. 2023. Update in: Elife. 2023 May 18;12:e86166. doi: 10.7554/eLife.86166. PMID: 36747846 Free PMC article. Updated. Preprint.
References
-
- Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR: Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96: 329–339, 1999 - PubMed
-
- Otani K, Kulkarni RN, Baldwin AC, Krutzfeldt J, Ueki K, Stoffel M, Kahn CR, Polonski KS: Reduced β-cell mass and altered glucose sensing impair insulin-secretory function in βIRKO mice. Am J Physiol 286: E41–E49, 2004 - PubMed
-
- Strivastava S, Goren HJ: Insulin constitutively secreted by β-cells is necessary for glucose-stimulated insulin secretion. Diabetes 52: 2049–2056, 2003 - PubMed
-
- Montell CK, Jones K, Hafen E, Rubin G: Rescue of the Drosophlia phototransduction mutation trp by germline transformation. Science 230: 1040–1043, 1985 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

