P bodies promote stress granule assembly in Saccharomyces cerevisiae
- PMID: 18981231
- PMCID: PMC2575786
- DOI: 10.1083/jcb.200807043
P bodies promote stress granule assembly in Saccharomyces cerevisiae
Abstract
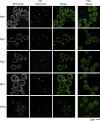
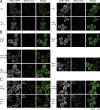
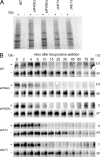
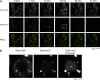
Recent results indicate that nontranslating mRNAs in eukaryotic cells exist in distinct biochemical states that accumulate in P bodies and stress granules, although the nature of interactions between these particles is unknown. We demonstrate in Saccharomyces cerevisiae that RNA granules with similar protein composition and assembly mechanisms as mammalian stress granules form during glucose deprivation. Stress granule assembly is dependent on P-body formation, whereas P-body assembly is independent of stress granule formation. This suggests that stress granules primarily form from mRNPs in preexisting P bodies, which is also supported by the kinetics of P-body and stress granule formation both in yeast and mammalian cells. These observations argue that P bodies are important sites for decisions of mRNA fate and that stress granules, at least in yeast, primarily represent pools of mRNAs stalled in the process of reentry into translation from P bodies.
Figures




Similar articles
-
Analyzing P-bodies and stress granules in Saccharomyces cerevisiae.Methods Enzymol. 2010;470:619-40. doi: 10.1016/S0076-6879(10)70025-2. Epub 2010 Mar 1. Methods Enzymol. 2010. PMID: 20946828
-
Acidic stress induces the formation of P-bodies, but not stress granules, with mild attenuation of bulk translation in Saccharomyces cerevisiae.Biochem J. 2012 Sep 1;446(2):225-33. doi: 10.1042/BJ20120583. Biochem J. 2012. PMID: 22686455
-
Stress-specific composition, assembly and kinetics of stress granules in Saccharomyces cerevisiae.J Cell Sci. 2011 Jan 15;124(Pt 2):228-39. doi: 10.1242/jcs.078444. Epub 2010 Dec 15. J Cell Sci. 2011. PMID: 21172806 Free PMC article.
-
Relationship of GW/P-bodies with stress granules.Adv Exp Med Biol. 2013;768:197-211. doi: 10.1007/978-1-4614-5107-5_12. Adv Exp Med Biol. 2013. PMID: 23224972 Free PMC article. Review.
-
Eukaryotic stress granules: the ins and outs of translation.Mol Cell. 2009 Dec 25;36(6):932-41. doi: 10.1016/j.molcel.2009.11.020. Mol Cell. 2009. PMID: 20064460 Free PMC article. Review.
Cited by
-
The DHH1/RCKp54 family of helicases: an ancient family of proteins that promote translational silencing.Biochim Biophys Acta. 2013 Aug;1829(8):817-23. doi: 10.1016/j.bbagrm.2013.03.006. Epub 2013 Mar 23. Biochim Biophys Acta. 2013. PMID: 23528737 Free PMC article. Review.
-
Stress granules, RNA-binding proteins and polyglutamine diseases: too much aggregation?Cell Death Dis. 2021 Jun 8;12(6):592. doi: 10.1038/s41419-021-03873-8. Cell Death Dis. 2021. PMID: 34103467 Free PMC article. Review.
-
PKA isoforms coordinate mRNA fate during nutrient starvation.J Cell Sci. 2012 Nov 1;125(Pt 21):5221-32. doi: 10.1242/jcs.111534. Epub 2012 Aug 16. J Cell Sci. 2012. PMID: 22899713 Free PMC article.
-
Evolutionarily conserved 5'-3' exoribonuclease Xrn1 accumulates at plasma membrane-associated eisosomes in post-diauxic yeast.PLoS One. 2015 Mar 26;10(3):e0122770. doi: 10.1371/journal.pone.0122770. eCollection 2015. PLoS One. 2015. PMID: 25811606 Free PMC article.
-
5' to 3' mRNA decay factors colocalize with Ty1 gag and human APOBEC3G and promote Ty1 retrotransposition.J Virol. 2010 May;84(10):5052-66. doi: 10.1128/JVI.02477-09. Epub 2010 Mar 10. J Virol. 2010. PMID: 20219921 Free PMC article.
References
-
- Abramoff, M.D., P.J. Magelhaes, and S.J. Ram. 2004. Image processing with ImageJ. Biophotonics Intl. 11:36–42.
-
- Anderson, P., and N. Kedersha. 2008. Stress granules: the Tao of RNA triage. Trends Biochem. Sci. 33:141–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

