Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells
- PMID: 18981114
- PMCID: PMC2706157
- DOI: 10.4049/jimmunol.181.10.6942
Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells
Abstract
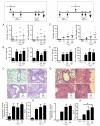
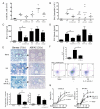
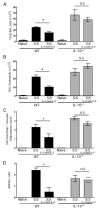
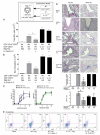
Regulatory T cells (Treg) play a decisive role in many diseases including asthma and allergen-induced lung inflammation. However, little progress has been made developing new therapeutic strategies for pulmonary disorders. In the current study we demonstrate that cytokine:antibody complexes of IL-2 and anti-IL-2 mAb reduce the severity of allergen-induced inflammation in the lung by expanding Tregs in vivo. Unlike rIL-2 or anti-IL-2 mAb treatment alone, IL-2:anti-IL-2 complexes dampened airway inflammation and eosinophilia while suppressing IL-5 and eotaxin-1 production. Mucus production, airway hyperresponsiveness to methacholine, and parenchymal tissue inflammation were also dramatically reduced following IL-2:anti-IL-2 treatment. The suppression in allergic airway disease was associated with a marked expansion of Tregs (IL-10(+)CD4(+)CD25(+) and Foxp3(+)CD4(+)CD25(+)) in the tissues, with a corresponding decrease in effector T cell responses. The ability of IL-2:anti-IL-2 complexes to suppress airway inflammation was dependent on Treg-derived IL-10, as IL-10(+/+), but not IL-10(-/-) Tregs, were capable of mediating the suppression. Furthermore, a therapeutic protocol using a model of established airway allergy highlighted the ability of IL-2:anti-IL-2 complexes to expand Tregs and prevent successive airway inflammation and airway hyperresponsiveness. This study suggests that endogenous Treg therapy may be a useful tool to combat the rising incidence of allergic airway disease.
Figures



Similar articles
-
Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent.J Exp Med. 2005 Dec 5;202(11):1539-47. doi: 10.1084/jem.20051166. Epub 2005 Nov 28. J Exp Med. 2005. PMID: 16314435 Free PMC article.
-
Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis.Circulation. 2012 Sep 4;126(10):1256-66. doi: 10.1161/CIRCULATIONAHA.112.099044. Epub 2012 Jul 31. Circulation. 2012. PMID: 22851544
-
Phenotype analyses of IL-10-producing Foxp3- CD4+ T cells increased by subcutaneous immunotherapy in allergic airway inflammation.Int Immunopharmacol. 2018 Aug;61:297-305. doi: 10.1016/j.intimp.2018.06.014. Epub 2018 Jun 14. Int Immunopharmacol. 2018. PMID: 29909233
-
Regulatory T cells and asthma.Clin Exp Allergy. 2009 Sep;39(9):1314-23. doi: 10.1111/j.1365-2222.2009.03301.x. Epub 2009 Jun 17. Clin Exp Allergy. 2009. PMID: 19538496 Review.
-
Regulatory T cells mediated immunomodulation during asthma: a therapeutic standpoint.J Transl Med. 2020 Dec 2;18(1):456. doi: 10.1186/s12967-020-02632-1. J Transl Med. 2020. PMID: 33267824 Free PMC article. Review.
Cited by
-
Regulatory T Cells, a Viable Target Against Airway Allergic Inflammatory Responses in Asthma.Front Immunol. 2022 Jun 10;13:902318. doi: 10.3389/fimmu.2022.902318. eCollection 2022. Front Immunol. 2022. PMID: 35757774 Free PMC article. Review.
-
Subsets of regulatory T cells and their roles in allergy.J Transl Med. 2014 May 12;12:125. doi: 10.1186/1479-5876-12-125. J Transl Med. 2014. PMID: 24886492 Free PMC article. Review.
-
Bufei Yishen Granules Combined with Acupoint Sticking Therapy Suppress Inflammation in Chronic Obstructive Pulmonary Disease Rats: Via JNK/p38 Signaling Pathway.Evid Based Complement Alternat Med. 2017;2017:1768243. doi: 10.1155/2017/1768243. Epub 2017 Oct 30. Evid Based Complement Alternat Med. 2017. PMID: 29234369 Free PMC article.
-
IL-2-Agonist-Induced IFN-γ Exacerbates Systemic Anaphylaxis in Food Allergen-Sensitized Mice.Front Immunol. 2020 Dec 11;11:596772. doi: 10.3389/fimmu.2020.596772. eCollection 2020. Front Immunol. 2020. PMID: 33362780 Free PMC article.
-
Interleukin 31 receptor α promotes smooth muscle cell contraction and airway hyperresponsiveness in asthma.Nat Commun. 2023 Dec 11;14(1):8207. doi: 10.1038/s41467-023-44040-1. Nat Commun. 2023. PMID: 38081868 Free PMC article.
References
-
- Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-γ. Arthritis Res. Ther. 2005;7:R402–R415. - PMC - PubMed
-
- Marazuela M, Garcia-Lopez MA, Figueroa-Vega N, de la Fuente H, Alvarado-Sanchez B, Monsivais-Urenda A, Sanchez-Madrid F, Gonzalez-Amaro R. Regulatory T cells in human autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 2006;91:3639–3646. - PubMed
-
- Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212–1218. - PubMed
-
- Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 2005;5:271–283. - PubMed
-
- Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

