The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo
- PMID: 18980173
- PMCID: PMC2612734
- DOI: 10.1002/ajh.21310
The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo
Abstract
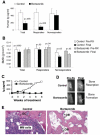
Multiple myeloma (MM), a hematologic malignancy of terminally differentiated plasma cells is closely associated with induction of osteolytic bone disease, induced by stimulation of osteoclastogenesis and suppression of osteoblastogenesis. The ubiquitin-proteasome pathway regulates differentiation of bone cells and MM cell growth. The proteasome inhibitor, bortezomib, is a clinical potent antimyeloma agent. The main goal of this study was to investigate the effect of bortezomib on myeloma-induced bone resorption and tumor growth in SCID-rab mice engrafted with MM cells from 16 patients. Antimyeloma response of bortezomib, which was evident in >50% of 16 experiments and resembled clinical response, was associated with significant increased bone mineral density (BMD) and osteoblast numbers, and reduced osteoclast numbers in myelomatous bones. This bone anabolic effect, which was also visualized on X-ray radiographs and confirmed by static and dynamic histomorphometric analyses, was unique to bortezomib and was not observed in hosts responding to melphalan, a chemotherapeutic drug widely used to treat MM. Bortezomib also increased BMD and osteoblasts number and reduced osteoclasts number in nonmyelomatous implanted bones. In vitro bortezomib directly suppressed human osteoclast formation and promoted maturation of osteoblasts. We conclude that bortezomib promotes bone formation in myelomatous and nonmyelomatous bones by simultaneously inhibiting osteoclastogenesis and stimulating osteoblastogenesis. As clinical and experimental studies indicate that bone disease is both a consequence and necessity of MM progression our results suggest and that bortezomib's effects on bone remodeling contribute to the antimyeloma efficacy of this drug.
Figures



Comment in
-
Effects of bortezomib on bone disease in multiple myeloma.Am J Hematol. 2009 Jan;84(1):1-2. doi: 10.1002/ajh.21324. Am J Hematol. 2009. PMID: 19030185 No abstract available.
Similar articles
-
Biological aspects of altered bone remodeling in multiple myeloma and possibilities of pharmacological intervention.Dan Med Bull. 2011 May;58(5):B4277. Dan Med Bull. 2011. PMID: 21535989 Review.
-
The effects of bortezomib on bone disease in patients with multiple myeloma.Cancer. 2014 Mar 1;120(5):618-23. doi: 10.1002/cncr.28481. Epub 2013 Nov 18. Cancer. 2014. PMID: 24249482 Review.
-
Consequences of daily administered parathyroid hormone on myeloma growth, bone disease, and molecular profiling of whole myelomatous bone.PLoS One. 2010 Dec 20;5(12):e15233. doi: 10.1371/journal.pone.0015233. PLoS One. 2010. PMID: 21188144 Free PMC article.
-
The proteasome inhibitor bortezomib inhibits FGF-2-induced reduction of TAZ levels in osteoblast-like cells.Eur J Haematol. 2010 Jul;85(1):68-75. doi: 10.1111/j.1600-0609.2010.01435.x. Epub 2010 Feb 23. Eur J Haematol. 2010. PMID: 20192985
-
The proteasome inhibitor bortezomib stimulates osteoblastic differentiation of human osteoblast precursors via upregulation of vitamin D receptor signalling.Eur J Haematol. 2013 Apr;90(4):263-72. doi: 10.1111/ejh.12069. Epub 2013 Feb 15. Eur J Haematol. 2013. PMID: 23311753
Cited by
-
Computational modeling of interactions between multiple myeloma and the bone microenvironment.PLoS One. 2011;6(11):e27494. doi: 10.1371/journal.pone.0027494. Epub 2011 Nov 8. PLoS One. 2011. PMID: 22110661 Free PMC article.
-
A retrospective study of skeletal and disease-free survival benefits of zoledronic acid therapy in patients with multiple myeloma treated with novel agents.Int J Clin Exp Med. 2013;6(1):30-8. Epub 2012 Nov 18. Int J Clin Exp Med. 2013. PMID: 23236556 Free PMC article.
-
Role of Bruton's tyrosine kinase in myeloma cell migration and induction of bone disease.Am J Hematol. 2013 Jun;88(6):463-71. doi: 10.1002/ajh.23433. Epub 2013 Mar 28. Am J Hematol. 2013. PMID: 23456977 Free PMC article.
-
The Proteasome Inhibitor Carfilzomib Suppresses Parathyroid Hormone-induced Osteoclastogenesis through a RANKL-mediated Signaling Pathway.J Biol Chem. 2015 Jul 3;290(27):16918-28. doi: 10.1074/jbc.M115.663963. Epub 2015 May 15. J Biol Chem. 2015. PMID: 25979341 Free PMC article.
-
Therapeutic effects of intrabone and systemic mesenchymal stem cell cytotherapy on myeloma bone disease and tumor growth.J Bone Miner Res. 2012 Aug;27(8):1635-48. doi: 10.1002/jbmr.1620. J Bone Miner Res. 2012. PMID: 22460389 Free PMC article.
References
-
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. - PubMed
-
- Barille-Nion S, Bataille R. New insights in myeloma-induced osteolysis. Leuk Lymphoma. 2003;44:1463–1467. - PubMed
-
- Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J Clin Oncol. 2005;23:8580–8587. - PubMed
-
- Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
