MacB ABC transporter is a dimer whose ATPase activity and macrolide-binding capacity are regulated by the membrane fusion protein MacA
- PMID: 18955484
- PMCID: PMC2613632
- DOI: 10.1074/jbc.M806964200
MacB ABC transporter is a dimer whose ATPase activity and macrolide-binding capacity are regulated by the membrane fusion protein MacA
Abstract
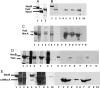
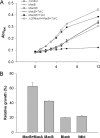
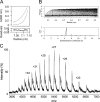
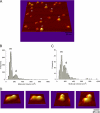
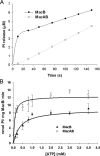
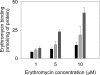
Gram-negative bacteria utilize specialized machinery to translocate drugs and protein toxins across the inner and outer membranes, consisting of a tripartite complex composed of an inner membrane secondary or primary active transporter (IMP), a periplasmic membrane fusion protein, and an outer membrane channel. We have investigated the assembly and function of the MacAB/TolC system that confers resistance to macrolides in Escherichia coli. The membrane fusion protein MacA not only stabilizes the tripartite assembly by interacting with both the inner membrane protein MacB and the outer membrane protein TolC, but also has a role in regulating the function of MacB, apparently increasing its affinity for both erythromycin and ATP. Analysis of the kinetic behavior of ATP hydrolysis indicated that MacA promotes and stabilizes the ATP-binding form of the MacB transporter. For the first time, we have established unambiguously the dimeric nature of a noncanonic ABC transporter, MacB that has an N-terminal nucleotide binding domain, by means of nondissociating mass spectrometry, analytical ultracentrifugation, and atomic force microscopy. Structural studies of ABC transporters indicate that ATP is bound between a pair of nucleotide binding domains to stabilize a conformation in which the substrate-binding site is outward-facing. Consequently, our data suggest that in the presence of ATP the same conformation of MacB is promoted and stabilized by MacA. Thus, MacA would facilitate the delivery of drugs by MacB to TolC by enhancing the binding of drugs to it and inducing a conformation of MacB that is primed and competent for binding TolC. Our structural studies are an important first step in understanding how the tripartite complex is assembled.
Figures
Similar articles
-
Role of ATP binding and hydrolysis in assembly of MacAB-TolC macrolide transporter.Mol Microbiol. 2012 Dec;86(5):1132-43. doi: 10.1111/mmi.12046. Epub 2012 Oct 12. Mol Microbiol. 2012. PMID: 23057817 Free PMC article.
-
The periplasmic membrane proximal domain of MacA acts as a switch in stimulation of ATP hydrolysis by MacB transporter.Mol Microbiol. 2011 Aug;81(4):937-51. doi: 10.1111/j.1365-2958.2011.07744.x. Epub 2011 Jul 4. Mol Microbiol. 2011. PMID: 21696464 Free PMC article.
-
Reconstitution of the Escherichia coli macrolide transporter: the periplasmic membrane fusion protein MacA stimulates the ATPase activity of MacB.Mol Microbiol. 2007 Feb;63(3):895-910. doi: 10.1111/j.1365-2958.2006.05549.x. Epub 2007 Jan 4. Mol Microbiol. 2007. PMID: 17214741
-
Antibiotic Resistance Mediated by the MacB ABC Transporter Family: A Structural and Functional Perspective.Front Microbiol. 2018 May 28;9:950. doi: 10.3389/fmicb.2018.00950. eCollection 2018. Front Microbiol. 2018. PMID: 29892271 Free PMC article. Review.
-
Structural and functional characteristics of the tripartite ABC transporter.Microbiology (Reading). 2022 Nov;168(11). doi: 10.1099/mic.0.001257. Microbiology (Reading). 2022. PMID: 36409601 Review.
Cited by
-
Molecular architecture of the bacterial tripartite multidrug efflux pump focusing on the adaptor bridging model.J Microbiol. 2015 Jun;53(6):355-64. doi: 10.1007/s12275-015-5248-4. Epub 2015 May 30. J Microbiol. 2015. PMID: 26025167 Review.
-
Assembly and channel opening of outer membrane protein in tripartite drug efflux pumps of Gram-negative bacteria.J Biol Chem. 2012 Apr 6;287(15):11740-50. doi: 10.1074/jbc.M111.329375. Epub 2012 Feb 3. J Biol Chem. 2012. PMID: 22308040 Free PMC article.
-
Identification of functional candidates amongst hypothetical proteins of Treponema pallidum ssp. pallidum.PLoS One. 2015 Apr 20;10(4):e0124177. doi: 10.1371/journal.pone.0124177. eCollection 2015. PLoS One. 2015. PMID: 25894582 Free PMC article.
-
How far can we go with structural mass spectrometry of protein complexes?J Am Soc Mass Spectrom. 2010 Apr;21(4):487-500. doi: 10.1016/j.jasms.2009.12.017. Epub 2010 Jan 4. J Am Soc Mass Spectrom. 2010. PMID: 20116283
-
Novel ATP-driven pathway of glycolipid export involving TolC protein.J Biol Chem. 2011 Nov 4;286(44):38202-38210. doi: 10.1074/jbc.M111.269332. Epub 2011 Sep 14. J Biol Chem. 2011. PMID: 21917923 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

