Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer
- PMID: 18954898
- PMCID: PMC2606929
- DOI: 10.1016/j.ygyno.2008.09.021
Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer
Abstract
Objectives: The invasive growth of circulating tumor cells (CTCs) propagates cancer metastasis. The aims of this study were to evaluate the association of invasive CTCs, detected by a novel cell invasion assay, with disease stage, CA-125 level and patient survival.
Methods: Peripheral blood samples from 71 patients undergoing evaluation for ovarian malignancy were assessed for the presence of invasive CTCs using a cell invasion assay that enriches and identifies tumor cells with a cell adhesion matrix (CAM). Invasive CTCs were identified as cells exhibiting CAM invasion (CAM+) and expressing standard epithelial markers (Epi+).
Results: 43 (60.6%) patients had detectable CTCs: 0/5 benign patients, 1/10 (10%) early stage, 39/52 (73.1%) late stage and 3/4 (75%) unstaged patients (p-value <0.001). CTC counts ranged from 0-149 CTCs/ml with stage III/IV patients exhibiting significantly higher mean counts (41.3 CTCs/ml) than stage I/II patients (6.0 CTCs/ml) and benign patients (0 CTCs/ml, p-value=0.001). A positive correlation between CTC count and CA-125 level was observed (Spearman correlation coefficient r=0.309, p-value=0.035). Kaplan-Meier curves revealed a significant decrease in disease-free survival in patients with detectable CTCs (median survival 15.0 months vs. 35.0 months, log-rank p-value=0.042). Tumor grade and tumor histology did not influence CTC detection.
Conclusions: Invasive CTCs can be detected in a majority of epithelial ovarian cancer patients and may predict shorter disease-free survival. Furthermore, higher CTC counts may reflect later stage disease and higher CA-125 levels.
Conflict of interest statement
Although we do not feel that the following information has biased our research in any capacity, we would like to disclose the following information:
Wen-Tien Chen is the founder of Vitatex Inc., the company who manufactures the cell invasion assay (Vita-Assay™) used in this work.
Tina Fan is the daughter-in-law of Wen-Tien Chen and served as a consultant to Vitatex Inc. for 9 months in 2006-2007. At the time she performed the work presented in this manuscript, she was no longer employed by Vitatex Inc., however her spouse maintains a position in the company.
Again, we would like to emphasize that despite these
Figures

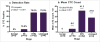


Similar articles
-
Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients.Gynecol Oncol. 2013 Nov;131(2):352-6. doi: 10.1016/j.ygyno.2013.08.006. Epub 2013 Aug 13. Gynecol Oncol. 2013. PMID: 23954902 Clinical Trial.
-
Detection of circulating tumor cells using manually performed immunocytochemistry (MICC) does not correlate with outcome in patients with early breast cancer - Results of the German SUCCESS-A- trial.BMC Cancer. 2016 Jul 7;16:401. doi: 10.1186/s12885-016-2454-3. BMC Cancer. 2016. PMID: 27387743 Free PMC article. Clinical Trial.
-
Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer.Gynecol Oncol. 2011 Sep;122(3):567-72. doi: 10.1016/j.ygyno.2011.05.028. Epub 2011 Jun 12. Gynecol Oncol. 2011. PMID: 21664658 Clinical Trial.
-
[Circulating tumor cells (CTCs)--clinical significance in patients with ovarian cancer].Ginekol Pol. 2012 Apr;83(4):291-4. Ginekol Pol. 2012. PMID: 22712262 Review. Polish.
-
Current and future role of circulating tumor cells in patients with epithelial ovarian cancer.Eur J Surg Oncol. 2016 Dec;42(12):1772-1779. doi: 10.1016/j.ejso.2016.05.010. Epub 2016 May 25. Eur J Surg Oncol. 2016. PMID: 27265041 Review.
Cited by
-
Circulating tumor cells in germ cell tumors: are those biomarkers of real prognostic value? A review.Clujul Med. 2016;89(2):203-11. doi: 10.15386/cjmed-570. Epub 2016 Apr 15. Clujul Med. 2016. PMID: 27152069 Free PMC article. Review.
-
Liquid biopsy in ovarian cancer in China and the world: current status and future perspectives.Front Oncol. 2023 Dec 19;13:1276085. doi: 10.3389/fonc.2023.1276085. eCollection 2023. Front Oncol. 2023. PMID: 38169730 Free PMC article. Review.
-
Liquid Biopsies for Ovarian Carcinoma: How Blood Tests May Improve the Clinical Management of a Deadly Disease.Cancers (Basel). 2019 Jun 4;11(6):774. doi: 10.3390/cancers11060774. Cancers (Basel). 2019. PMID: 31167492 Free PMC article. Review.
-
The propensity of invasive circulating tumor cells (iCTCs) in metastatic progression and therapeutic responsiveness.Cancer Med. 2019 Jul;8(8):3864-3874. doi: 10.1002/cam4.2218. Epub 2019 May 21. Cancer Med. 2019. PMID: 31115187 Free PMC article.
-
Current methodologies to detect circulating tumor cells: a focus on ovarian cancer.Am J Cancer Res. 2021 Sep 15;11(9):4111-4126. eCollection 2021. Am J Cancer Res. 2021. PMID: 34659879 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar;58(2):71–96. - PubMed
-
- Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006 Nov;95 1:S161–S192. - PubMed
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004 Dec 9;351(24):2519–29. - PubMed
-
- Cain JM, Ellis GK, Collins C, Greer BE, Tamimi HK, Figge DC, et al. Bone marrow involvement in epithelial ovarian cancer by immunocytochemical assessment. Gynecologic Oncology. 1990 Sep;38(3):442–5. - PubMed
-
- Braun S, Schindlbeck C, Hepp F, Janni W, Kentenich C, Riethmuller G, et al. Occult tumor cells in bone marrow of patients with locoregionally restricted ovarian cancer predict early distant metastatic relapse. Journal of Clinical Oncology. 2001 Jan 15;19(2):368–75. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

