Reprogramming primordial germ cells into pluripotent stem cells
- PMID: 18953407
- PMCID: PMC2567847
- DOI: 10.1371/journal.pone.0003531
Reprogramming primordial germ cells into pluripotent stem cells
Abstract
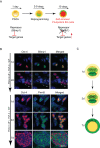
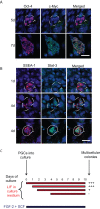
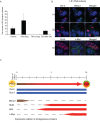
Background: Specification of primordial germ cells (PGCs) results in the conversion of pluripotent epiblast cells into monopotent germ cell lineage. Blimp1/Prmt5 complex plays a critical role in the specification and maintenance of the early germ cell lineage. However, PGCs can be induced to dedifferentiate back to a pluripotent state as embryonic germ (EG) cells when exposed to exogenous signaling molecules, FGF-2, LIF and SCF.
Methodology and principal findings: Here we show that Trichostatin A (TSA), an inhibitor of histone deacetylases, is a highly potent agent that can replace FGF-2 to induce dedifferentiation of PGCs into EG cells. A key early event during dedifferentiation of PGCs in response to FGF-2 or TSA is the down-regulation of Blimp1, which reverses and apparently relieves the cell fate restriction imposed by it. Notably, the targets of Blimp1, which include c-Myc and Klf-4, which represent two of the key factors known to promote reprogramming of somatic cells to pluripotent state, are up-regulated. We also found early activation of the LIF/Stat-3 signaling pathway with the translocation of Stat-3 into the nucleus. By contrast, while Prmt5 is retained in EG cells, it translocates from the nucleus to the cytoplasm where it probably has an independent role in regulating pluripotency.
Conclusions/significance: We propose that dedifferentiation of PGCs into EG cells may provide significant mechanistic insights on early events associated with reprogramming of committed cells to a pluripotent state.
Conflict of interest statement
Figures
Similar articles
-
The germ cell determinant Blimp1 is not required for derivation of pluripotent stem cells.Cell Stem Cell. 2012 Jul 6;11(1):110-7. doi: 10.1016/j.stem.2012.02.023. Cell Stem Cell. 2012. PMID: 22770244 Free PMC article.
-
Rebuilding pluripotency from primordial germ cells.Stem Cell Reports. 2013 Jun 4;1(1):66-78. doi: 10.1016/j.stemcr.2013.03.004. eCollection 2013. Stem Cell Reports. 2013. PMID: 24052943 Free PMC article.
-
Integrative Analysis of the Acquisition of Pluripotency in PGCs Reveals the Mutually Exclusive Roles of Blimp-1 and AKT Signaling.Stem Cell Reports. 2015 Jul 14;5(1):111-24. doi: 10.1016/j.stemcr.2015.05.007. Epub 2015 Jun 4. Stem Cell Reports. 2015. PMID: 26050930 Free PMC article.
-
In or out stemness: comparing growth factor signalling in mouse embryonic stem cells and primordial germ cells.Curr Stem Cell Res Ther. 2009 May;4(2):87-97. doi: 10.2174/157488809788167391. Curr Stem Cell Res Ther. 2009. PMID: 19442193 Review.
-
How to make a primordial germ cell.Development. 2014 Jan;141(2):245-52. doi: 10.1242/dev.098269. Development. 2014. PMID: 24381195 Review.
Cited by
-
Present state and future perspectives of using pluripotent stem cells in toxicology research.Arch Toxicol. 2011 Feb;85(2):79-117. doi: 10.1007/s00204-010-0641-6. Epub 2011 Jan 12. Arch Toxicol. 2011. PMID: 21225242 Free PMC article. Review.
-
Protein arginine methyltransferase 5 functions in opposite ways in the cytoplasm and nucleus of prostate cancer cells.PLoS One. 2012;7(8):e44033. doi: 10.1371/journal.pone.0044033. Epub 2012 Aug 27. PLoS One. 2012. PMID: 22952863 Free PMC article.
-
Chromatin connections to pluripotency and cellular reprogramming.Cell. 2011 Jun 10;145(6):835-50. doi: 10.1016/j.cell.2011.05.019. Cell. 2011. PMID: 21663790 Free PMC article. Review.
-
Cell-intrinsic reprogramming capability: gain or loss of pluripotency in germ cells.Reprod Med Biol. 2012 Jun 19;12(1):1-14. doi: 10.1007/s12522-012-0131-z. eCollection 2013 Jan. Reprod Med Biol. 2012. PMID: 29699125 Free PMC article. Review.
-
Specification and epigenetic programming of the human germ line.Nat Rev Genet. 2016 Oct;17(10):585-600. doi: 10.1038/nrg.2016.88. Epub 2016 Aug 30. Nat Rev Genet. 2016. PMID: 27573372 Review.
References
-
- Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;439:207–213. - PubMed
-
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, et al. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;6:623–630. - PubMed
-
- Matsui Y, Zsebo K, Hogan BLM. Derivation of pluripotent embryonic stem cells from murine primordial germ cells. Cell. 1992;70:841–847. - PubMed
-
- Resnick JL, Bixler LS, Cheng L, Donovan PJ. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. - PubMed
-
- Durcova-Hills G, Adams IR, Barton SC, Surani MA, McLaren A. The role of exogenous fibroblast growth factor-2 on the reprogramming of primordial germ cells into pluripotent stem cells. Stem Cells. 2006;24:1441–1449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

