Axonal transport and the delivery of pre-synaptic components
- PMID: 18950710
- PMCID: PMC2653082
- DOI: 10.1016/j.conb.2008.10.003
Axonal transport and the delivery of pre-synaptic components
Abstract
The mechanisms for delivering components to nerve terminals are diverse and highly regulated. The diversity of kinesin motors alone is insufficient to account for the specificity of delivery. Additional specificity and control are contributed by adaptor proteins and associated regulatory molecules. The interaction of cargos with these complexes can confer distinct behaviors on the transport of synaptic organelles. The rich regulatory mechanisms of transport that are only now emerging as the cargo-motor complexes are defined and subsequent local events that regulate their dynamic relationship are examined. Here we review recent studies of kinesin-related axonal transport of three crucial synaptic components, Piccolo-bassoon Transport Vesicles (PTVs), Synaptic Vesicle Precursors (SVPs), and mitochondria, and the mechanisms that modulate their transport.
Conflict of interest statement
The authors have no conflicts of interest with the publication of this article.
Figures
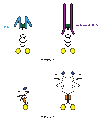
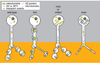
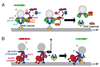

Similar articles
-
Single-axonal organelle analysis method reveals new protein-motor associations.ACS Chem Neurosci. 2013 Feb 20;4(2):277-84. doi: 10.1021/cn300136y. Epub 2012 Dec 7. ACS Chem Neurosci. 2013. PMID: 23421679 Free PMC article.
-
Dynein light chain regulates axonal trafficking and synaptic levels of Bassoon.J Cell Biol. 2009 Apr 20;185(2):341-55. doi: 10.1083/jcb.200807155. J Cell Biol. 2009. PMID: 19380881 Free PMC article.
-
Molecular organization and assembly of the presynaptic active zone of neurotransmitter release.Results Probl Cell Differ. 2006;43:49-68. doi: 10.1007/400_012. Results Probl Cell Differ. 2006. PMID: 17068967 Review.
-
The Conserved IgSF9 Protein Borderless Regulates Axonal Transport of Presynaptic Components and Color Vision in Drosophila.J Neurosci. 2019 Aug 28;39(35):6817-6828. doi: 10.1523/JNEUROSCI.0075-19.2019. Epub 2019 Jun 24. J Neurosci. 2019. PMID: 31235647 Free PMC article.
-
Building a Terminal: Mechanisms of Presynaptic Development in the CNS.Neuroscientist. 2016 Aug;22(4):372-91. doi: 10.1177/1073858415596131. Epub 2015 Jul 24. Neuroscientist. 2016. PMID: 26208860 Review.
Cited by
-
α-Synuclein oligomers impair neuronal microtubule-kinesin interplay.J Biol Chem. 2013 Jul 26;288(30):21742-54. doi: 10.1074/jbc.M113.451815. Epub 2013 Jun 6. J Biol Chem. 2013. PMID: 23744071 Free PMC article.
-
Basic mechanisms of neurodegeneration: a critical update.J Cell Mol Med. 2010 Mar;14(3):457-87. doi: 10.1111/j.1582-4934.2010.01010.x. Epub 2010 Jan 11. J Cell Mol Med. 2010. PMID: 20070435 Free PMC article. Review.
-
Presynaptic Autophagy and the Connection With Neurotransmission.Front Cell Dev Biol. 2021 Dec 17;9:790721. doi: 10.3389/fcell.2021.790721. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34988081 Free PMC article. Review.
-
Traffic control: regulation of kinesin motors.Nat Rev Mol Cell Biol. 2009 Nov;10(11):765-77. doi: 10.1038/nrm2782. Nat Rev Mol Cell Biol. 2009. PMID: 19851335 Review.
-
Disruption of mitochondrial DNA replication in Drosophila increases mitochondrial fast axonal transport in vivo.PLoS One. 2009 Nov 17;4(11):e7874. doi: 10.1371/journal.pone.0007874. PLoS One. 2009. PMID: 19924234 Free PMC article.
References
-
- De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Role of Axonal Transport in Neurodegenerative Diseases. Annu Rev Neurosci. 2008;31:151–173. - PubMed
-
- Stokin GB, Goldstein LS. Axonal transport and Alzheimer's disease. Annu Rev Biochem. 2006;75:607–627. - PubMed
-
- Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–191. - PubMed
-
- Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 2005;490:209–219. - PubMed
-
- Ibanez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17:519–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

