Adoptive immunotherapy: good habits instilled at youth have long-term benefits
- PMID: 18949448
- PMCID: PMC3809041
- DOI: 10.1007/s12026-008-8070-9
Adoptive immunotherapy: good habits instilled at youth have long-term benefits
Abstract
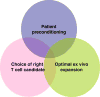
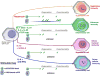
Many recent advances in basic cell biology and immunology are a harbinger of progress in adoptive cell therapy (ACT) including (1) the finding that host lymphodepletion enhances engraftment and efficacy, (2) the recognition that in vitro T cell functions may not correlate with in vivo efficacy, and (3) the development of advanced ex vivo culture methods to expand lymphocytes to therapeutically effective numbers. In this article, we focus on the development of artificial antigen presenting cells (aAPCs) in our laboratory and their applicability to augment ACT protocols. We also describe how aAPCs can be used to broaden ACT to treat patients with a wide variety of cancers, chronic infectious diseases, and autoimmune manifestations.
Figures


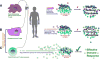
Similar articles
-
Transient stimulation expands superior antitumor T cells for adoptive therapy.JCI Insight. 2017 Jan 26;2(2):e89580. doi: 10.1172/jci.insight.89580. JCI Insight. 2017. PMID: 28138559 Free PMC article.
-
Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy.Trends Immunol. 2005 Feb;26(2):111-7. doi: 10.1016/j.it.2004.12.003. Trends Immunol. 2005. PMID: 15668127 Free PMC article. Review.
-
Generation of Antitumor T Cells For Adoptive Cell Therapy With Artificial Antigen Presenting Cells.J Immunother. 2020 Apr;43(3):79-88. doi: 10.1097/CJI.0000000000000306. J Immunother. 2020. PMID: 31834208 Free PMC article.
-
Role of memory T cell subsets for adoptive immunotherapy.Semin Immunol. 2016 Feb;28(1):28-34. doi: 10.1016/j.smim.2016.02.001. Epub 2016 Mar 11. Semin Immunol. 2016. PMID: 26976826 Free PMC article. Review.
-
Artificial antigen-presenting cells: the booster for the obtaining of functional adoptive cells.Cell Mol Life Sci. 2024 Aug 31;81(1):378. doi: 10.1007/s00018-024-05412-y. Cell Mol Life Sci. 2024. PMID: 39215816 Free PMC article. Review.
Cited by
-
Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer.Nat Rev Cancer. 2010 Mar;10(3):213-21. doi: 10.1038/nrc2804. Epub 2010 Feb 19. Nat Rev Cancer. 2010. PMID: 20168320 Review.
-
Exploiting IL-17-producing CD4+ and CD8+ T cells to improve cancer immunotherapy in the clinic.Cancer Immunol Immunother. 2016 Mar;65(3):247-59. doi: 10.1007/s00262-016-1797-6. Epub 2016 Jan 29. Cancer Immunol Immunother. 2016. PMID: 26825102 Free PMC article. Review.
-
CAR T cells for infection, autoimmunity and allotransplantation.Nat Rev Immunol. 2018 Oct;18(10):605-616. doi: 10.1038/s41577-018-0042-2. Nat Rev Immunol. 2018. PMID: 30046149 Free PMC article. Review.
-
Augmented lymphocyte expansion from solid tumors with engineered cells for costimulatory enhancement.J Immunother. 2011 Nov-Dec;34(9):651-61. doi: 10.1097/CJI.0b013e31823284c3. J Immunother. 2011. PMID: 21989413 Free PMC article.
-
The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma.Front Immunol. 2021 Mar 5;12:609421. doi: 10.3389/fimmu.2021.609421. eCollection 2021. Front Immunol. 2021. PMID: 33767695 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

