Parkin deficiency increases vulnerability to inflammation-related nigral degeneration
- PMID: 18945890
- PMCID: PMC2603252
- DOI: 10.1523/JNEUROSCI.3001-08.2008
Parkin deficiency increases vulnerability to inflammation-related nigral degeneration
Abstract
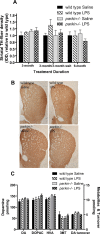
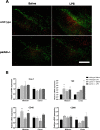
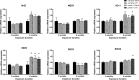
The loss of nigral dopaminergic (DA) neurons in idiopathic Parkinson's disease (PD) is believed to result from interactions between genetic susceptibility and environmental factors. Evidence that inflammatory processes modulate PD risk comes from prospective studies that suggest that higher plasma concentrations of a number of proinflammatory cytokines correlate with an increased risk of developing PD and chronic nonsteroidal anti-inflammatory drug regimens reduce the incidence of PD. Although loss-of-function mutations in the parkin gene cause early-onset familial PD, Parkin-deficient (parkin-/-) mice do not display nigrostriatal pathway degeneration, suggesting that a genetic factor is not sufficient, and an environmental trigger may be needed to cause nigral DA neuron loss. To test the hypothesis that parkin-/- mice require an inflammatory stimulus to develop nigral DA neuron loss, low-dose lipopolysaccaride (LPS) was administered intraperitoneally for prolonged periods. Quantitative real-time PCR and immunofluorescence labeling of inflammatory markers indicated that this systemic LPS treatment regimen triggered persistent neuroinflammation in wild-type and parkin-/- mice. Although inflammatory and oxidative stress responses to the inflammation regimen did not differ significantly between the two genotypes, only parkin-/- mice displayed subtle fine-motor deficits and selective loss of DA neurons in substantia nigra. Therefore, our studies suggest that loss of Parkin function increases the vulnerability of nigral DA neurons to inflammation-related degeneration. This new model of nigral DA neuron loss may enable identification of early biomarkers of degeneration and aid in preclinical screening efforts to identify compounds that can halt or delay the progressive degeneration of the nigrostriatal pathway.
Figures


Similar articles
-
Parkin-knockout mice did not display increased vulnerability to intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).Neurotox Res. 2013 Aug;24(2):280-7. doi: 10.1007/s12640-013-9389-0. Epub 2013 Apr 16. Neurotox Res. 2013. PMID: 23588969
-
Analysis of inflammation-related nigral degeneration and locomotor function in DJ-1(-/-) mice.J Neuroinflammation. 2013 Apr 28;10:50. doi: 10.1186/1742-2094-10-50. J Neuroinflammation. 2013. PMID: 23622116 Free PMC article.
-
Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice.J Neurochem. 2009 Nov;111(3):696-702. doi: 10.1111/j.1471-4159.2009.06350.x. Epub 2009 Aug 19. J Neurochem. 2009. PMID: 19694908 Free PMC article.
-
[Etiology and pathogenesis of Parkinson's disease: from mitochondrial dysfunctions to familial Parkinson's disease].Rinsho Shinkeigaku. 2004 Apr-May;44(4-5):241-62. Rinsho Shinkeigaku. 2004. PMID: 15287506 Review. Japanese.
-
Inflammatory process as a determinant factor for the degeneration of substantia nigra dopaminergic neurons.J Neural Transm (Vienna). 2005 Jan;112(1):111-9. doi: 10.1007/s00702-004-0121-3. Epub 2004 Mar 19. J Neural Transm (Vienna). 2005. PMID: 15599609 Review.
Cited by
-
Pathways to Parkinsonism Redux: convergent pathobiological mechanisms in genetics of Parkinson's disease.Hum Mol Genet. 2015 Oct 15;24(R1):R32-44. doi: 10.1093/hmg/ddv236. Epub 2015 Jun 22. Hum Mol Genet. 2015. PMID: 26101198 Free PMC article. Review.
-
Post-translational modification and mitochondrial function in Parkinson's disease.Front Mol Neurosci. 2024 Jan 11;16:1329554. doi: 10.3389/fnmol.2023.1329554. eCollection 2023. Front Mol Neurosci. 2024. PMID: 38273938 Free PMC article. Review.
-
PINK1 stimulates interleukin-1β-mediated inflammatory signaling via the positive regulation of TRAF6 and TAK1.Cell Mol Life Sci. 2012 Oct;69(19):3301-15. doi: 10.1007/s00018-012-1004-7. Epub 2012 May 29. Cell Mol Life Sci. 2012. PMID: 22643835 Free PMC article.
-
Parkin-knockout mice did not display increased vulnerability to intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).Neurotox Res. 2013 Aug;24(2):280-7. doi: 10.1007/s12640-013-9389-0. Epub 2013 Apr 16. Neurotox Res. 2013. PMID: 23588969
-
The Challenge and Opportunity to Diagnose Parkinson's Disease in Midlife.Front Neurol. 2019 Dec 17;10:1328. doi: 10.3389/fneur.2019.01328. eCollection 2019. Front Neurol. 2019. PMID: 31920948 Free PMC article. Review.
References
-
- Abbott LC, Jacobowitz DM. Development of calretinin-immunoreactive unipolar brush-like cells and an afferent pathway to the embryonic and early postnatal mouse cerebellum. Anat Embryol (Berl) 1995;191:541–559. - PubMed
-
- Aggarwal BB, Samanta A, Feldmann M. TNF receptors. In: Oppenheim JJ, Feldmann M, editors. Cytokine reference. London: Academic; 2000. pp. 1620–1632.
-
- Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord. 1998;13:221–227. - PubMed
-
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson's disease. J Neurochem. 2002;82:615–624. - PubMed
-
- Cassarino DS, Halvorsen EM, Swerdlow RH, Abramova NN, Parker WD, Jr, Sturgill TW, Bennett JP., Jr Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-kappaB in cellular models of Parkinson's disease. J Neurochem. 2000;74:1384–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
