Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors
- PMID: 18941233
- PMCID: PMC3104927
- DOI: 10.4049/jimmunol.181.9.6427
Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors
Abstract
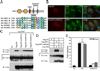
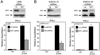
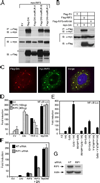
DNA-dependent activator of IFN regulatory factors (IRF; DAI, also known as ZBP1 or DLM-1) is a cytosolic DNA sensor that initiates IRF3 and NF-kappaB pathways leading to activation of type I IFNs (IFNalpha, IFNbeta) and other cytokines. In this study, induction of NF-kappaB is shown to depend on the adaptor receptor-interacting protein kinase (RIP)1, acting via a RIP homotypic interaction motif (RHIM)-dependent interaction with DAI. DAI binds to and colocalizes with endogenous RIP1 at characteristic cytoplasmic granules. Suppression of RIP1 expression by RNAi abrogates NF-kappaB activation as well as IFNbeta induction by immunostimulatory DNA. DAI also interacts with RIP3 and this interaction potentiates DAI-mediated activation of NF-kappaB, implicating RIP3 in regulating this RHIM-dependent pathway. The role of DAI in activation of NF-kappaB in response to immunostimulatory DNA appears to be analogous to sensing of dsRNA by TLR3 in that both pathways involve RHIM-dependent signaling that is mediated via RIP1, reinforcing a central role for this adaptor in innate sensing of intracellular microbes.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

Similar articles
-
DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB.EMBO Rep. 2009 Aug;10(8):916-22. doi: 10.1038/embor.2009.109. Epub 2009 Jul 10. EMBO Rep. 2009. PMID: 19590578 Free PMC article.
-
DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA.Cell Host Microbe. 2012 Mar 15;11(3):290-7. doi: 10.1016/j.chom.2012.01.016. Cell Host Microbe. 2012. PMID: 22423968 Free PMC article.
-
Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif.J Immunol. 2005 Apr 15;174(8):4942-52. doi: 10.4049/jimmunol.174.8.4942. J Immunol. 2005. PMID: 15814722
-
DNA sensors in innate immune system.Uirusu. 2008 Jun;58(1):37-46. doi: 10.2222/jsv.58.37. Uirusu. 2008. PMID: 19122387 Review.
-
CARD tricks: controlling the interactions of CARD6 with RICK and microtubules.Cell Cycle. 2006 Apr;5(8):797-800. doi: 10.4161/cc.5.8.2635. Epub 2006 Apr 17. Cell Cycle. 2006. PMID: 16582588 Review.
Cited by
-
RIP3: a molecular switch for necrosis and inflammation.Genes Dev. 2013 Aug 1;27(15):1640-9. doi: 10.1101/gad.223321.113. Genes Dev. 2013. PMID: 23913919 Free PMC article. Review.
-
ZBP1: A Powerful Innate Immune Sensor and Double-Edged Sword in Host Immunity.Int J Mol Sci. 2022 Sep 6;23(18):10224. doi: 10.3390/ijms231810224. Int J Mol Sci. 2022. PMID: 36142136 Free PMC article. Review.
-
Acetaminophen modulates the transcriptional response to recombinant interferon-beta.PLoS One. 2010 Jun 9;5(6):e11031. doi: 10.1371/journal.pone.0011031. PLoS One. 2010. PMID: 20544007 Free PMC article.
-
ADAR1 and ZBP1 in innate immunity, cell death, and disease.Trends Immunol. 2023 Mar;44(3):201-216. doi: 10.1016/j.it.2023.01.001. Epub 2023 Jan 27. Trends Immunol. 2023. PMID: 36710220 Free PMC article. Review.
-
Viral Z-RNA triggers ZBP1-dependent cell death.Curr Opin Virol. 2021 Dec;51:134-140. doi: 10.1016/j.coviro.2021.10.004. Epub 2021 Oct 21. Curr Opin Virol. 2021. PMID: 34688984 Free PMC article. Review.
References
-
- Takaoka A, Taniguchi T. Cytosolic DNA recognition for triggering innate immune responses. Adv. Drug Delivery Rev. 2007;60:847–857. - PubMed
-
- Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:525–532. - PubMed
-
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. - PubMed
-
- Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. - PubMed
-
- Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv. Drug Delivery Rev. 2008;60:795–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

