Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells
- PMID: 18941193
- PMCID: PMC2753458
- DOI: 10.4049/jimmunol.181.9.6038
Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells
Abstract
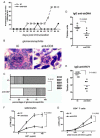
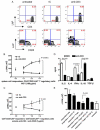
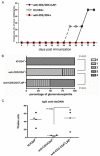
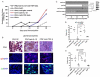
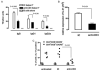
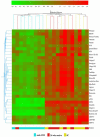
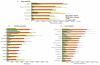
Lupus is an Ab-mediated autoimmune disease. One of the potential contributors to the development of systemic lupus erythematosus is a defect in naturally occurring CD4(+)CD25(+) regulatory T cells. Thus, the generation of inducible regulatory T cells that can control autoantibody responses is a potential avenue for the treatment of systemic lupus erythematosus. We have found that nasal administration of anti-CD3 mAb attenuated lupus development as well as arrested ongoing lupus in two strains of lupus-prone mice. Nasal anti-CD3 induced a CD4(+)CD25(-)latency-associated peptide (LAP)(+) regulatory T cell that secreted high levels of IL-10 and suppressed disease in vivo via IL-10- and TFG-beta-dependent mechanisms. Disease suppression also occurred following adoptive transfer of CD4(+)CD25(-)LAP(+) regulatory T cells from nasal anti-CD3-treated animals to lupus-prone mice. Animals treated with nasal anti-CD3 had less glomerulonephritis and diminished levels of autoantibodies as measured by both ELISA and autoantigen microarrays. Nasal anti-CD3 affected the function of CD4(+)ICOS(+)CXCR5(+) follicular helper T cells that are required for autoantibody production. CD4(+)ICOS(+)CXCR5(+) follicular helper T cells express high levels of IL-17 and IL-21 and these cytokines were down-regulated by nasal anti-CD3. Our results demonstrate that nasal anti-CD3 induces CD4(+)CD25(-)LAP(+) regulatory T cells that suppress lupus in mice and that it is associated with down-regulation of T cell help for autoantibody production.
Figures

Similar articles
-
Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25-LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells.Lupus. 2009 Jun;18(7):586-96. doi: 10.1177/0961203308100511. Lupus. 2009. PMID: 19433458 Free PMC article.
-
The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo.J Immunol. 2005 Aug 15;175(4):2340-8. doi: 10.4049/jimmunol.175.4.2340. J Immunol. 2005. PMID: 16081804
-
Egr2 and Egr3 in regulatory T cells cooperatively control systemic autoimmunity through Ltbp3-mediated TGF-β3 production.Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):E8131-E8140. doi: 10.1073/pnas.1611286114. Epub 2016 Nov 30. Proc Natl Acad Sci U S A. 2016. PMID: 27911796 Free PMC article.
-
IL-10 producing regulatory and helper T-cells in systemic lupus erythematosus.Semin Immunol. 2019 Aug;44:101330. doi: 10.1016/j.smim.2019.101330. Epub 2019 Nov 15. Semin Immunol. 2019. PMID: 31735515 Review.
-
Follicular Helper T Cells in Systemic Lupus Erythematosus.Front Immunol. 2018 Aug 3;9:1793. doi: 10.3389/fimmu.2018.01793. eCollection 2018. Front Immunol. 2018. PMID: 30123218 Free PMC article. Review.
Cited by
-
Oral Administration of OKT3 MAb to Patients with NASH, Promotes Regulatory T-cell Induction, and Alleviates Insulin Resistance: Results of a Phase IIa Blinded Placebo-Controlled Trial.J Clin Immunol. 2015 May;35(4):399-407. doi: 10.1007/s10875-015-0160-6. Epub 2015 Apr 17. J Clin Immunol. 2015. PMID: 25876706 Clinical Trial.
-
IL-17 in systemic lupus erythematosus.J Biomed Biotechnol. 2010;2010:943254. doi: 10.1155/2010/943254. Epub 2010 Apr 6. J Biomed Biotechnol. 2010. PMID: 20379379 Free PMC article. Review.
-
Understanding the development and function of T follicular helper cells.Cell Mol Immunol. 2010 May;7(3):190-7. doi: 10.1038/cmi.2010.24. Epub 2010 Apr 12. Cell Mol Immunol. 2010. PMID: 20383172 Free PMC article. Review.
-
Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH.Clin Exp Immunol. 2018 Sep;193(3):275-283. doi: 10.1111/cei.13159. Clin Exp Immunol. 2018. PMID: 29920654 Free PMC article. Review.
-
Inducing and Administering Tregs to Treat Human Disease.Front Immunol. 2016 Jan 22;6:654. doi: 10.3389/fimmu.2015.00654. eCollection 2015. Front Immunol. 2016. PMID: 26834735 Free PMC article. Review.
References
-
- Ravirajan CT, Rahman MA, Papadaki L, Griffiths MH, Kalsi J, Martin AC, Ehrenstein MR, Latchman DS, Isenberg DA. Genetic, structural, and functional properties of an IgG DNA-binding monoclonal antibody from a lupus patient with nephritis. Eur. J. Immunol. 1998;28:339–350. - PubMed
-
- Ehrenstein MR, Katz DR, Griffiths MH, Papadaki L, Winkler TH, Kalden JR, Isenberg DA. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995;48:705–711. - PubMed
-
- Okamura M, Kanayama Y, Amastu K, Negoro N, Kohda S, Takeda T, Inoue T. Significance of enzyme linked immunosorbent assay (ELISA) for antibodies to double stranded and single stranded DNA in patients with lupus nephritis: correlation with severity of renal histology. Ann. Rheum. Dis. 1993;52:14–20. - PMC - PubMed
-
- Rahman A. Autoantibodies, lupus, and the science of sabotage. Rheumatology. 2004;43:1326–1336. - PubMed
-
- Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Lane PJ. Co-stimulation and selection for T-cell help for germinal centres: the role of CD28 and OX40. Immunol. Today. 2000;21:333–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

