Covalent and noncovalent intermediates of an NAD utilizing enzyme, human CD38
- PMID: 18940667
- PMCID: PMC2607045
- DOI: 10.1016/j.chembiol.2008.08.007
Covalent and noncovalent intermediates of an NAD utilizing enzyme, human CD38
Abstract
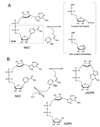
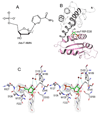
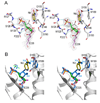
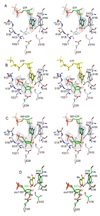
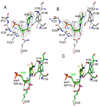
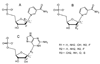
Enzymatic utilization of nicotinamide adenine dinucleotide (NAD) has increasingly been shown to have fundamental roles in gene regulation, signal transduction, and protein modification. Many of the processes require the cleavage of the nicotinamide moiety from the substrate and the formation of a reactive intermediate. Using X-ray crystallography, we show that human CD38, an NAD-utilizing enzyme, is capable of catalyzing the cleavage reactions through both covalent and noncovalent intermediates, depending on the substrate used. The covalent intermediate is resistant to further attack by nucleophiles, resulting in mechanism-based enzyme inactivation. The noncovalent intermediate is stabilized mainly through H-bond interactions, but appears to remain reactive. Our structural results favor the proposal of a noncovalent intermediate during normal enzymatic utilization of NAD by human CD38 and provide structural insights into the design of covalent and noncovalent inhibitors targeting NAD-utilization pathways.
Figures
Similar articles
-
Dynamic conformations of the CD38-mediated NAD cyclization captured in a single crystal.J Mol Biol. 2011 Jan 28;405(4):1070-8. doi: 10.1016/j.jmb.2010.11.044. Epub 2010 Dec 8. J Mol Biol. 2011. PMID: 21134381 Free PMC article.
-
Insights into the mechanism of bovine CD38/NAD+glycohydrolase from the X-ray structures of its Michaelis complex and covalently-trapped intermediates.PLoS One. 2012;7(4):e34918. doi: 10.1371/journal.pone.0034918. Epub 2012 Apr 18. PLoS One. 2012. PMID: 22529956 Free PMC article.
-
Structural basis for the mechanistic understanding of human CD38-controlled multiple catalysis.J Biol Chem. 2006 Oct 27;281(43):32861-9. doi: 10.1074/jbc.M606365200. Epub 2006 Sep 2. J Biol Chem. 2006. PMID: 16951430
-
CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions.Curr Pharm Des. 2009;15(1):57-63. doi: 10.2174/138161209787185788. Curr Pharm Des. 2009. PMID: 19149603 Free PMC article. Review.
-
Role of CD38 in Adipose Tissue: Tuning Coenzyme Availability?Nutrients. 2021 Oct 23;13(11):3734. doi: 10.3390/nu13113734. Nutrients. 2021. PMID: 34835990 Free PMC article. Review.
Cited by
-
Structural basis for enzymatic evolution from a dedicated ADP-ribosyl cyclase to a multifunctional NAD hydrolase.J Biol Chem. 2009 Oct 2;284(40):27637-45. doi: 10.1074/jbc.M109.031005. Epub 2009 Jul 28. J Biol Chem. 2009. PMID: 19640846 Free PMC article.
-
The choice of sequence homologs included in multiple sequence alignments has a dramatic impact on evolutionary conservation analysis.Bioinformatics. 2019 Jan 1;35(1):12-19. doi: 10.1093/bioinformatics/bty523. Bioinformatics. 2019. PMID: 29947739 Free PMC article.
-
Dynamic conformations of the CD38-mediated NAD cyclization captured in a single crystal.J Mol Biol. 2011 Jan 28;405(4):1070-8. doi: 10.1016/j.jmb.2010.11.044. Epub 2010 Dec 8. J Mol Biol. 2011. PMID: 21134381 Free PMC article.
-
Design, synthesis and SAR studies of NAD analogues as potent inhibitors towards CD38 NADase.Molecules. 2014 Sep 29;19(10):15754-67. doi: 10.3390/molecules191015754. Molecules. 2014. PMID: 25268725 Free PMC article.
-
Diastereocontrolled electrophilic fluorinations of 2-deoxyribonolactone: syntheses of all corresponding 2-deoxy-2-fluorolactones and 2'-deoxy-2'-fluoro-NAD+s.J Org Chem. 2009 Aug 21;74(16):5779-89. doi: 10.1021/jo900637f. J Org Chem. 2009. PMID: 19958035 Free PMC article.
References
-
- Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem. 1995;270:30327–30333. - PubMed
-
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. - PubMed
-
- Bull HG, Ferraz JP, Cordes EH, Ribbi A, Apitzcastro R. Concerning Mechanism of Enzymatic and Non-Enzymatic Hydrolysis of Nicotinamide Nucleotide Coenzymes. Journal of Biological Chemistry. 1978;253:5186–5192. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

