IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury
- PMID: 18938163
- PMCID: PMC2632606
- DOI: 10.1016/j.expneurol.2008.09.014
IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury
Abstract
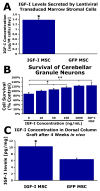
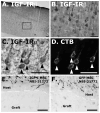
An unmet challenge of spinal cord injury research is the identification of mechanisms that promote regeneration of corticospinal motor axons. Recently it was reported that IGF-I promotes corticospinal axon growth during nervous system development. We therefore investigated whether IGF-I also promotes regeneration or survival of adult lesioned corticospinal neurons. Adult Fischer 344 rats underwent C3 dorsal column transections followed by grafts of IGF-I-secreting marrow stromal cell grafts into the lesion cavity. IGF-I secreting cell grafts promoted growth of raphespinal and cerulospinal axons, but not corticospinal axons, into the lesion/graft site. We then examined whether IGF-I-secreting cell grafts promote corticospinal motor neuron survival or axon growth in a subcortical axotomy model. IGF-I expression coupled with infusion of the IGF binding protein inhibitor NBI-31772 significantly prevented corticospinal motor neuron death (93% cell survival compared to 49% in controls, P<0.05), but did not promote corticospinal axon regeneration. Coincident with observed effects of IGF-I on corticospinal survival but not growth, expression of IGF-I receptors was restricted to the somal compartment and not the axon of adult corticospinal motor neurons. Thus, whereas IGF-I influences corticospinal axonal growth during development, its application to sites of adult spinal cord injury or subcortical axotomy fails to promote corticospinal axonal regeneration under conditions that are sufficient to prevent corticospinal cell death and promote the growth of other supraspinal axons. We conclude that developmental patterns of growth factor responsiveness are not simply recapitulated after adult injury, potentially due to post-natal shifts in patterns of IGF-I receptor expression.
Figures



Similar articles
-
Dependence of regenerated sensory axons on continuous neurotrophin-3 delivery.J Neurosci. 2012 Sep 19;32(38):13206-20. doi: 10.1523/JNEUROSCI.5041-11.2012. J Neurosci. 2012. PMID: 22993437 Free PMC article.
-
Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells.Exp Neurol. 1997 Dec;148(2):444-52. doi: 10.1006/exnr.1997.6704. Exp Neurol. 1997. PMID: 9417824
-
Neurotrophism without neurotropism: BDNF promotes survival but not growth of lesioned corticospinal neurons.J Comp Neurol. 2001 Aug 6;436(4):456-70. doi: 10.1002/cne.1080. J Comp Neurol. 2001. PMID: 11447589
-
The Dorsal Column Lesion Model of Spinal Cord Injury and Its Use in Deciphering the Neuron-Intrinsic Injury Response.Dev Neurobiol. 2018 Oct;78(10):926-951. doi: 10.1002/dneu.22601. Epub 2018 May 11. Dev Neurobiol. 2018. PMID: 29717546 Free PMC article. Review.
-
Applications of Proteomics to Nerve Regeneration Research.In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review.
Cited by
-
Promoting axon regeneration in the central nervous system by increasing PI3-kinase signaling.Neural Regen Res. 2022 Jun;17(6):1172-1182. doi: 10.4103/1673-5374.327324. Neural Regen Res. 2022. PMID: 34782551 Free PMC article. Review.
-
Understanding the axonal response to injury by in vivo imaging in the mouse spinal cord: A tale of two branches.Exp Neurol. 2019 Aug;318:277-285. doi: 10.1016/j.expneurol.2019.04.008. Epub 2019 Apr 12. Exp Neurol. 2019. PMID: 30986398 Free PMC article. Review.
-
Therapeutic potential of IGF-I on hippocampal neurogenesis and function during aging.Neurogenesis (Austin). 2016 Dec 20;4(1):e1259709. doi: 10.1080/23262133.2016.1259709. eCollection 2017. Neurogenesis (Austin). 2016. PMID: 28405590 Free PMC article.
-
Gene Manipulation Strategies to Identify Molecular Regulators of Axon Regeneration in the Central Nervous System.Front Cell Neurosci. 2017 Aug 7;11:231. doi: 10.3389/fncel.2017.00231. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28824380 Free PMC article. Review.
-
Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation.Proc Natl Acad Sci U S A. 2009 Apr 28;106(17):7215-20. doi: 10.1073/pnas.0810624106. Epub 2009 Apr 9. Proc Natl Acad Sci U S A. 2009. PMID: 19359495 Free PMC article.
References
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. - PubMed
-
- Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19:5574–5581. - PubMed
-
- Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–730. - PubMed
-
- Bilak MM, Kuncl RW. Delayed application of IGF-I and GDNF can rescue already injured postnatal motor neurons. Neuroreport. 2001;12:2531–2535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

