Tropomodulin1 is required in the heart but not the yolk sac for mouse embryonic development
- PMID: 18927466
- PMCID: PMC2744601
- DOI: 10.1161/CIRCRESAHA.108.178749
Tropomodulin1 is required in the heart but not the yolk sac for mouse embryonic development
Abstract
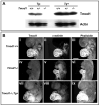
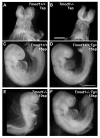
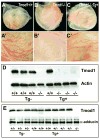
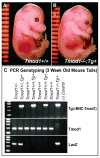
Tropomodulin (Tmod)1 caps the pointed ends of actin filaments in sarcomeres of striated muscle myofibrils and in the erythrocyte membrane skeleton. Targeted deletion of mouse Tmod1 leads to defects in cardiac development, fragility of primitive erythroid cells, and an absence of yolk sac vasculogenesis, followed by embryonic lethality at embryonic day 9.5. The Tmod1-null embryonic hearts do not undergo looping morphogenesis and the cardiomyocytes fail to assemble striated myofibrils with regulated F-actin lengths. To test whether embryonic lethality of Tmod1 nulls results from defects in cardiac myofibrillogenesis and development or from erythroid cell fragility and subsequent defects in yolk sac vasculogenesis, we expressed Tmod1 specifically in the myocardium of the Tmod1-null mice under the control of the alpha-myosin heavy chain promoter Tg(alphaMHC-Tmod1). In contrast to Tmod1-null embryos, which fail to undergo cardiac looping and have defective yolk sac vasculogenesis, both cardiac and yolk sac morphology of Tmod1(-/-Tg(alphaMHC-Tmod1)) embryos are normal at embryonic day 9.5. Tmod1(-/-Tg(alphaMHC-Tmod1)) embryos develop into viable and fertile mice, indicating that expression of Tmod1 in the heart is sufficient to rescue the Tmod1-null embryonic defects. Thus, although loss of Tmod1 results in myriad defects and embryonic lethality, the Tmod1(-/-) primary defect is in the myocardium. Moreover, Tmod1 is not required in erythrocytes for viability, nor do the Tmod1(-/-) fragile primitive erythroid cells affect cardiac development, yolk sac vasculogenesis, or viability in the mouse.
Conflict of interest statement
Figures

Similar articles
-
Aberrant myofibril assembly in tropomodulin1 null mice leads to aborted heart development and embryonic lethality.J Cell Biol. 2003 Dec 8;163(5):1033-44. doi: 10.1083/jcb.200308164. Epub 2003 Dec 1. J Cell Biol. 2003. PMID: 14657235 Free PMC article.
-
E-Tmod capping of actin filaments at the slow-growing end is required to establish mouse embryonic circulation.Am J Physiol Heart Circ Physiol. 2003 May;284(5):H1827-38. doi: 10.1152/ajpheart.00947.2002. Epub 2003 Jan 23. Am J Physiol Heart Circ Physiol. 2003. PMID: 12543641
-
Transgenic Expression of the Formin Protein Fhod3 Selectively in the Embryonic Heart: Role of Actin-Binding Activity of Fhod3 and Its Sarcomeric Localization during Myofibrillogenesis.PLoS One. 2016 Feb 5;11(2):e0148472. doi: 10.1371/journal.pone.0148472. eCollection 2016. PLoS One. 2016. PMID: 26848968 Free PMC article.
-
Analysis of homozygous TGF beta 1 null mouse embryos demonstrates defects in yolk sac vasculogenesis and hematopoiesis.Ann N Y Acad Sci. 1995 Mar 27;752:300-8. doi: 10.1111/j.1749-6632.1995.tb17439.x. Ann N Y Acad Sci. 1995. PMID: 7755275 Review. No abstract available.
-
Capping actin filament growth: tropomodulin in muscle and nonmuscle cells.Soc Gen Physiol Ser. 1997;52:79-89. Soc Gen Physiol Ser. 1997. PMID: 9210222 Review.
Cited by
-
Formation, contraction, and mechanotransduction of myofribrils in cardiac development: clues from genetics.Biochem Res Int. 2012;2012:504906. doi: 10.1155/2012/504906. Epub 2012 Jun 10. Biochem Res Int. 2012. PMID: 22720160 Free PMC article.
-
Tropomodulin 1 Regulation of Actin Is Required for the Formation of Large Paddle Protrusions Between Mature Lens Fiber Cells.Invest Ophthalmol Vis Sci. 2016 Aug 1;57(10):4084-99. doi: 10.1167/iovs.16-19949. Invest Ophthalmol Vis Sci. 2016. PMID: 27537257 Free PMC article.
-
Tropomodulin 1-null mice have a mild spherocytic elliptocytosis with appearance of tropomodulin 3 in red blood cells and disruption of the membrane skeleton.Blood. 2010 Oct 7;116(14):2590-9. doi: 10.1182/blood-2010-02-268458. Epub 2010 Jun 28. Blood. 2010. PMID: 20585041 Free PMC article.
-
Characterizing interaction forces between actin and proteins of the tropomodulin family reveals the presence of the N-terminal actin-binding site in leiomodin.Arch Biochem Biophys. 2018 Jan 15;638:18-26. doi: 10.1016/j.abb.2017.12.005. Epub 2017 Dec 6. Arch Biochem Biophys. 2018. PMID: 29223925 Free PMC article.
-
Dynamic regulation of sarcomeric actin filaments in striated muscle.Cytoskeleton (Hoboken). 2010 Nov;67(11):677-92. doi: 10.1002/cm.20476. Cytoskeleton (Hoboken). 2010. PMID: 20737540 Free PMC article. Review.
References
-
- Fischer RS, Fowler VM. Tropomodulins: life at the slow end. Trends Cell Biol. 2003;13:593–601. - PubMed
-
- Fowler VM. Regulation of actin filament length in erythrocytes and striated muscle. Curr Opin Cell Biol. 1996;8:86–96. - PubMed
-
- Chu X, Chen J, Reedy MC, Vera C, Sung KL, Sung LA. E-Tmod capping of actin filaments at the slow-growing end is required to establish mouse embryonic circulation. Am J Physiol Heart Circ Physiol. 2003;284:H1827–1838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

