Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection
- PMID: 18922870
- PMCID: PMC2593304
- DOI: 10.1128/JVI.01215-08
Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection
Abstract
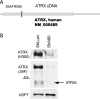
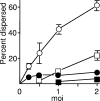
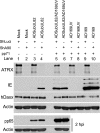
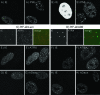
The human cytomegalovirus (HCMV) tegument protein pp71, encoded by gene UL82, stimulates viral immediate-early (IE) transcription. pp71 interacts with the cellular protein hDaxx at nuclear domain 10 (ND10) sites, resulting in the reversal of hDaxx-mediated repression of viral transcription. We demonstrate that pp71 displaces an hDaxx-binding protein, ATRX, from ND10 prior to any detectable effects on hDaxx itself and that this event contributes to the role of pp71 in alleviating repression. Introduction of pp71 into cells by transfection, infection with a pp71-expressing herpes simplex virus type 1 vector, or by generation of transformed cell lines promoted the rapid relocation of ATRX from ND10 to the nucleoplasm without alteration of hDaxx levels or localization. A pp71 mutant protein unable to interact with hDaxx did not affect the intranuclear distribution of ATRX. Infection with HCMV at a high multiplicity of infection resulted in rapid displacement of ATRX from ND10, the effect being observed maximally by 2 h after adsorption, whereas infection with the UL82-null HCMV mutant ADsubUL82 did not affect ATRX localization even at 7 h postinfection. Cell lines depleted of ATRX by transduction with shRNA-expressing lentiviruses supported increased IE gene expression and virus replication after infection with ADsubUL82, demonstrating that ATRX has a role in repressing IE transcription. The results show that ATRX, in addition to hDaxx, is a component of cellular intrinsic defenses that limit HCMV IE transcription and that displacement of ATRX from ND10 by pp71 is important for the efficient initiation of viral gene expression.
Figures



Similar articles
-
Stimulation of the Replication of ICP0-Null Mutant Herpes Simplex Virus 1 and pp71-Deficient Human Cytomegalovirus by Epstein-Barr Virus Tegument Protein BNRF1.J Virol. 2016 Oct 14;90(21):9664-9673. doi: 10.1128/JVI.01224-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535048 Free PMC article.
-
Role of the cellular protein hDaxx in human cytomegalovirus immediate-early gene expression.J Gen Virol. 2006 May;87(Pt 5):1113-1121. doi: 10.1099/vir.0.81566-0. J Gen Virol. 2006. PMID: 16603511
-
Insertion of an EYFP-pp71 (UL82) coding sequence into the human cytomegalovirus genome results in a recombinant virus with enhanced viral growth.J Virol. 2008 Nov;82(21):10543-55. doi: 10.1128/JVI.01006-08. Epub 2008 Aug 20. J Virol. 2008. PMID: 18715911 Free PMC article.
-
Expanding the Known Functional Repertoire of the Human Cytomegalovirus pp71 Protein.Front Cell Infect Microbiol. 2020 Mar 12;10:95. doi: 10.3389/fcimb.2020.00095. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32226778 Free PMC article. Review.
-
Intrinsic cellular defense mechanisms targeting human cytomegalovirus.Virus Res. 2011 May;157(2):128-33. doi: 10.1016/j.virusres.2010.10.002. Epub 2010 Oct 8. Virus Res. 2011. PMID: 20934469 Review.
Cited by
-
Virion factors that target Daxx to overcome intrinsic immunity.J Virol. 2013 Oct;87(19):10412-22. doi: 10.1128/JVI.00425-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864634 Free PMC article. Review.
-
Human cytomegalovirus riding the cell cycle.Med Microbiol Immunol. 2015 Jun;204(3):409-19. doi: 10.1007/s00430-015-0396-z. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776080 Review.
-
The Role of Nuclear Antiviral Factors against Invading DNA Viruses: The Immediate Fate of Incoming Viral Genomes.Viruses. 2016 Oct 22;8(10):290. doi: 10.3390/v8100290. Viruses. 2016. PMID: 27782081 Free PMC article. Review.
-
Tegument Proteins of Kaposi's Sarcoma-Associated Herpesvirus and Related Gamma-Herpesviruses.Front Microbiol. 2012 Mar 15;3:98. doi: 10.3389/fmicb.2012.00098. eCollection 2012. Front Microbiol. 2012. PMID: 22435068 Free PMC article.
-
Distinct temporal roles for the promyelocytic leukaemia (PML) protein in the sequential regulation of intracellular host immunity to HSV-1 infection.PLoS Pathog. 2018 Jan 8;14(1):e1006769. doi: 10.1371/journal.ppat.1006769. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29309427 Free PMC article.
References
-
- Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 27439-55. - PubMed
-
- Argentaro, A., J.-C. Yang, L. Chapman, M. S. Kowalczyk, R. J. Gibbons, D. R. HIggs, D. Neuhaus, and D. Rhodes. 2007. Structural consequences of disease-causing mutations in the ATRX-DNMT3L (ADD) domain of the chromatin-associated protein ATRX. Proc. Natl. Acad. Sci. USA 10411939-11944. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

