A role for SNX5 in the regulation of macropinocytosis
- PMID: 18854019
- PMCID: PMC2576169
- DOI: 10.1186/1471-2121-9-58
A role for SNX5 in the regulation of macropinocytosis
Abstract
Background: The mechanisms and components that regulate macropinocytosis are poorly understood. Here we have investigated the role of sorting nexin 5 (SNX5) in the regulation of macropinocytic activity.
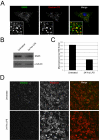
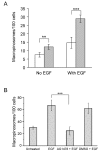
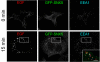
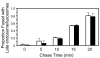
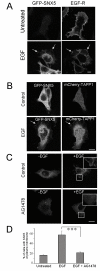
Results: SNX5 is abundantly expressed in macrophages, cells very active in macropinocytosis, and is recruited onto newly-formed macropinosomes. LPS treatment of bone marrow-derived macrophages resulted in a 2.5 fold decrease in macropinosome formation that correlates with a reduction in the levels of SNX5. To investigate the relationship between SNX5 levels and macropinocytic activity we examined the formation of macropinosomes in HEK-FlpIn cells stably expressing GFP-SNX5. Constitutive macropinocytosis was increased approximately 2 fold in HEK-GFP-SNX5 cells compared with parental HEK-FlpIn cells. Furthermore, EGF stimulation resulted in a significant increase in macropinocytosis and there was also a 2.0 fold increase in the generation of macropinosomes in HEK-GFP-SNX5 cells compared with parental HEK-FlpIn cells. SNX5, which interacts specifically with PtdIns(3)P and PtdIns(3,4)P2 through its PX domain, was recruited to regions on the plasma membrane containing EGF receptor or positive for PtdIns(3,4)P2 as detected with the PH domain of TAPP1. Treatment with AG1478, an EGF receptor specific tyrosine kinase inhibitor, prevented the recruitment of SNX5 to the cytosolic face of the plasma membrane and inhibited the formation of macropinosomes in response to EGF treatment.
Conclusion: Based on these data, we propose that SNX5 requires the generation of phosphoinositides for recruitment to the plasma membrane and, moreover, influences the level of macropinocytic activity.
Figures
Similar articles
-
Sorting nexin 5 is localized to a subdomain of the early endosomes and is recruited to the plasma membrane following EGF stimulation.J Cell Sci. 2004 Dec 15;117(Pt 26):6413-24. doi: 10.1242/jcs.01561. Epub 2004 Nov 23. J Cell Sci. 2004. PMID: 15561769
-
Sorting nexin 5 selectively regulates dorsal-ruffle-mediated macropinocytosis in primary macrophages.J Cell Sci. 2015 Dec 1;128(23):4407-19. doi: 10.1242/jcs.174359. Epub 2015 Oct 12. J Cell Sci. 2015. PMID: 26459636
-
Visualisation of macropinosome maturation by the recruitment of sorting nexins.J Cell Sci. 2006 Oct 1;119(Pt 19):3967-80. doi: 10.1242/jcs.03167. Epub 2006 Sep 12. J Cell Sci. 2006. PMID: 16968745
-
The Phox (PX) domain proteins and membrane traffic.Biochim Biophys Acta. 2006 Aug;1761(8):878-96. doi: 10.1016/j.bbalip.2006.04.011. Epub 2006 May 6. Biochim Biophys Acta. 2006. PMID: 16782399 Review.
-
Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides.J Cell Mol Med. 2007 Jul-Aug;11(4):670-84. doi: 10.1111/j.1582-4934.2007.00062.x. J Cell Mol Med. 2007. PMID: 17760832 Free PMC article. Review.
Cited by
-
Comparative Proteomic and Phosphoproteomic Analyses Reveal Molecular Signatures of Myocardial Infarction and Transverse Aortic Constriction in Aged Mouse Models.Cardiol Res Pract. 2024 Oct 28;2024:9395213. doi: 10.1155/2024/9395213. eCollection 2024. Cardiol Res Pract. 2024. PMID: 39502510 Free PMC article.
-
Arf5-mediated regulation of mTORC1 at the plasma membrane.Mol Biol Cell. 2023 Apr 1;34(4):ar23. doi: 10.1091/mbc.E22-07-0302. Epub 2023 Feb 3. Mol Biol Cell. 2023. PMID: 36735494 Free PMC article.
-
The association of lipid transfer protein VPS13A with endosomes is mediated by sorting nexin SNX5.Life Sci Alliance. 2023 Mar 28;6(6):e202201852. doi: 10.26508/lsa.202201852. Print 2023 Jun. Life Sci Alliance. 2023. PMID: 36977596 Free PMC article.
-
Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry.Part Fibre Toxicol. 2013 Feb 6;10:2. doi: 10.1186/1743-8977-10-2. Part Fibre Toxicol. 2013. PMID: 23388071 Free PMC article.
-
Albumin and mammalian cell culture: implications for biotechnology applications.Cytotechnology. 2010 Jan;62(1):1-16. doi: 10.1007/s10616-010-9263-3. Epub 2010 Apr 6. Cytotechnology. 2010. PMID: 20373019 Free PMC article.
References
-
- Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. - PubMed
-
- Racoosin EL, Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102:867–880. - PubMed
-
- Sun P, Yamamoto H, Suetsugu S, Miki H, Takenawa T, Endo T. Small GTPase Rah/Rab34 is associated with membrane ruffles and macropinosomes and promotes macropinosome formation. J Biol Chem. 2003;278:4063–4071. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

