Mgr3p and Mgr1p are adaptors for the mitochondrial i-AAA protease complex
- PMID: 18843051
- PMCID: PMC2592643
- DOI: 10.1091/mbc.e08-01-0103
Mgr3p and Mgr1p are adaptors for the mitochondrial i-AAA protease complex
Abstract
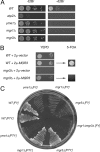
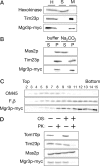
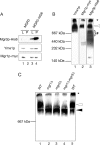
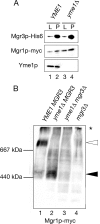
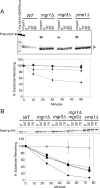
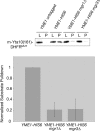
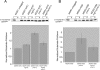
By screening yeast knockouts for their dependence upon the mitochondrial genome, we identified Mgr3p, a protein that associates with the i-AAA protease complex in the mitochondrial inner membrane. Mgr3p and Mgr1p, another i-AAA-interacting protein, form a subcomplex that bind to the i-AAA subunit Yme1p. We find that loss of Mgr3p, like the lack of Mgr1p, reduces proteolysis by Yme1p. Mgr3p and Mgr1p can bind substrate even in the absence of Yme1p, and both proteins are needed for maximal binding of an unfolded substrate by the i-AAA complex. We speculate that Mgr3p and Mgr1p function in an adaptor complex that targets substrates to the i-AAA protease for degradation.
Figures

Similar articles
-
A genomewide screen for petite-negative yeast strains yields a new subunit of the i-AAA protease complex.Mol Biol Cell. 2006 Jan;17(1):213-26. doi: 10.1091/mbc.e05-06-0585. Epub 2005 Nov 2. Mol Biol Cell. 2006. PMID: 16267274 Free PMC article.
-
Substrate recognition by AAA+ ATPases: distinct substrate binding modes in ATP-dependent protease Yme1 of the mitochondrial intermembrane space.Mol Cell Biol. 2007 Apr;27(7):2476-85. doi: 10.1128/MCB.01721-06. Epub 2007 Jan 29. Mol Cell Biol. 2007. PMID: 17261594 Free PMC article.
-
A AAA ATPase Cdc48 with a cofactor Ubx2 facilitates ubiquitylation of a mitochondrial fusion-promoting factor Fzo1 for proteasomal degradation.J Biochem. 2020 Mar 1;167(3):279-286. doi: 10.1093/jb/mvz104. J Biochem. 2020. PMID: 31804690
-
ATP-dependent proteases controlling mitochondrial function in the yeast Saccharomyces cerevisiae.Cell Mol Life Sci. 1999 Nov 30;56(9-10):825-42. doi: 10.1007/s000180050029. Cell Mol Life Sci. 1999. PMID: 11212342 Free PMC article. Review.
-
Mitochondrial AAA proteases--towards a molecular understanding of membrane-bound proteolytic machines.Biochim Biophys Acta. 2012 Jan;1823(1):49-55. doi: 10.1016/j.bbamcr.2011.09.015. Epub 2011 Oct 6. Biochim Biophys Acta. 2012. PMID: 22001671 Review.
Cited by
-
Antagonistic effects of mitochondrial matrix and intermembrane space proteases on yeast aging.BMC Biol. 2022 Jul 12;20(1):160. doi: 10.1186/s12915-022-01352-w. BMC Biol. 2022. PMID: 35820914 Free PMC article.
-
Current Understanding of Temperature Stress-Responsive Chloroplast FtsH Metalloproteases.Int J Mol Sci. 2021 Nov 9;22(22):12106. doi: 10.3390/ijms222212106. Int J Mol Sci. 2021. PMID: 34829988 Free PMC article. Review.
-
FTSH4 and OMA1 mitochondrial proteases reduce moderate heat stress-induced protein aggregation.Plant Physiol. 2021 Oct 5;187(2):769-786. doi: 10.1093/plphys/kiab296. Plant Physiol. 2021. PMID: 34608962 Free PMC article.
-
Polymerase delta-interacting protein 38 (PDIP38) modulates the stability and activity of the mitochondrial AAA+ protease CLPXP.Commun Biol. 2020 Nov 6;3(1):646. doi: 10.1038/s42003-020-01358-6. Commun Biol. 2020. PMID: 33159171 Free PMC article.
-
Novel IM-associated protein Tim54 plays a role in the mitochondrial import of internal signal-containing proteins in Trypanosoma brucei.Biol Cell. 2021 Jan;113(1):39-57. doi: 10.1111/boc.202000054. Epub 2020 Nov 9. Biol Cell. 2021. PMID: 33084070 Free PMC article.
References
-
- Adams A., Gottschling D., Kaiser C., Stearns T. Methods in yeast genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; 1997.
-
- Arnold I., Wagner-Ecker M., Ansorge W., Langer T. Evidence for a novel mitochondria-to-nucleus signalling pathway in respiring cells lacking i-AAA protease and the ABC-transporter Mdl1. Gene. 2006;367:74–88. - PubMed
-
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu. Rev. Cell Biol. 1988;4:289–333. - PubMed
-
- Augustin S., Nolden M., Muller S., Hardt O., Arnold I., Langer T. Characterization of peptides released from mitochondria: evidence for constant proteolysis and peptide efflux. J. Biol. Chem. 2005;280:2691–2699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

