Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner
- PMID: 18842721
- PMCID: PMC2593340
- DOI: 10.1128/JVI.01329-08
Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner
Abstract
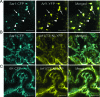
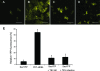
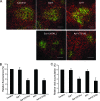
Single-stranded positive-sense RNA viruses induce the biogenesis of cytoplasmic membranous vesicles, where viral replication takes place. However, the mechanism underlying this characteristic vesicular proliferation remains poorly understood. Previously, a 6-kDa potyvirus membrane protein (6K) was shown to interact with the endoplasmic reticulum (ER) and to induce the formation of the membranous vesicles. In this study, the involvement of the early secretory pathway in the formation of the 6K-induced vesicles was investigated in planta. By means of live-cell imaging, it was found that the 6K protein was predominantly colocalized with Sar1, Sec23, and Sec24, which are known markers of ER exit sites (ERES). The localization of 6K at ERES was prevented by the coexpression of a dominant-negative mutant of Sar1 that disables the COPII activity or by the coexpression of a mutant of Arf1 that disrupts the COPI complex. The secretion of a soluble secretory marker targeting the apoplast was arrested at the level of the ER in cells overexpressing 6K or infected by a potyvirus. This blockage of protein trafficking out of the ER by 6K and the distribution of 6K toward the ERES may account for the aggregation of the 6K-bound vesicles. Finally, virus infection was reduced when the accumulation of 6K at ERES was inhibited by impairing either the COPI or COPII complex. Taken together, these results imply that the cellular COPI and COPII coating machineries are involved in the biogenesis of the potyvirus 6K vesicles at the ERES for viral-genome replication.
Figures
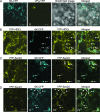
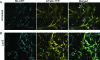
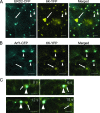
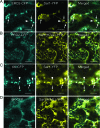
Similar articles
-
Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication.J Virol. 2010 Jan;84(2):799-809. doi: 10.1128/JVI.01824-09. Epub 2009 Nov 11. J Virol. 2010. PMID: 19906931 Free PMC article.
-
Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites.Plant Cell. 2005 May;17(5):1513-31. doi: 10.1105/tpc.104.026757. Epub 2005 Apr 1. Plant Cell. 2005. PMID: 15805489 Free PMC article.
-
In tobacco leaf epidermal cells, the integrity of protein export from the endoplasmic reticulum and of ER export sites depends on active COPI machinery.Plant J. 2006 Apr;46(1):95-110. doi: 10.1111/j.1365-313X.2006.02675.x. Plant J. 2006. PMID: 16553898
-
ARF1 and SAR1 GTPases in endomembrane trafficking in plants.Int J Mol Sci. 2013 Sep 5;14(9):18181-99. doi: 10.3390/ijms140918181. Int J Mol Sci. 2013. PMID: 24013371 Free PMC article. Review.
-
The role of ADP-ribosylation factor and SAR1 in vesicular trafficking in plants.Biochim Biophys Acta. 2004 Jul 1;1664(1):9-30. doi: 10.1016/j.bbamem.2004.04.005. Biochim Biophys Acta. 2004. PMID: 15238254 Review.
Cited by
-
DEVELOPMENTALLY REGULATED PLASMA MEMBRANE PROTEIN of Nicotiana benthamiana contributes to potyvirus movement and transports to plasmodesmata via the early secretory pathway and the actomyosin system.Plant Physiol. 2015 Feb;167(2):394-410. doi: 10.1104/pp.114.252734. Epub 2014 Dec 24. Plant Physiol. 2015. PMID: 25540331 Free PMC article.
-
Perception of double-stranded RNA in plant antiviral immunity.Mol Plant Pathol. 2019 Sep;20(9):1203-1210. doi: 10.1111/mpp.12798. Epub 2019 Apr 3. Mol Plant Pathol. 2019. PMID: 30942534 Free PMC article. Review.
-
Subcellular localization and rearrangement of endoplasmic reticulum by Brome mosaic virus capsid protein.J Virol. 2011 Mar;85(6):2953-63. doi: 10.1128/JVI.02020-10. Epub 2011 Jan 5. J Virol. 2011. PMID: 21209103 Free PMC article.
-
Ultrastructural Analysis of Cells From Bell Pepper (Capsicum annuum) Infected With Bell Pepper Endornavirus.Front Plant Sci. 2020 Apr 28;11:491. doi: 10.3389/fpls.2020.00491. eCollection 2020. Front Plant Sci. 2020. PMID: 32411163 Free PMC article.
-
Contribution of host intracellular transport machineries to intercellular movement of turnip mosaic virus.PLoS Pathog. 2013;9(10):e1003683. doi: 10.1371/journal.ppat.1003683. Epub 2013 Oct 3. PLoS Pathog. 2013. PMID: 24098128 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

