Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes
- PMID: 18829989
- PMCID: PMC2584129
- DOI: 10.2337/db08-0872
Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes
Abstract
Objective: To establish the mechanism of the phenotypic switch of adipose tissue macrophages (ATMs) from an alternatively activated (M2a) to a classically activated (M1) phenotype with obesity.
Research design and methods: ATMs from lean and obese (high-fat diet-fed) C57Bl/6 mice were analyzed by a combination of flow cytometry, immunofluorescence, and expression analysis for M2a and M1 genes. Pulse labeling of ATMs with PKH26 assessed the recruitment rate of ATMs to spatially distinct regions.
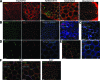
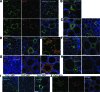
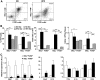
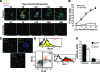
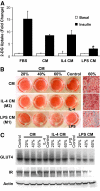
Results: Resident ATMs in lean mice express the M2a marker macrophage galactose N-acetyl-galactosamine specific lectin 1 (MGL1) and localize to interstitial spaces between adipocytes independent of CCR2 and CCL2. With diet-induced obesity, MGL1(+) ATMs remain in interstitial spaces, whereas a population of MGL1(-)CCR2(+) ATMs with high M1 and low M2a gene expression is recruited to clusters surrounding necrotic adipocytes. Pulse labeling showed that the rate of recruitment of new macrophages to MGL1(-) ATM clusters is significantly faster than that of interstitial MGL1(+) ATMs. This recruitment is attenuated in Ccr2(-/-) mice. M2a- and M1-polarized macrophages produced different effects on adipogenesis and adipocyte insulin sensitivity in vitro.
Conclusions: The shift in the M2a/M1 ATM balance is generated by spatial and temporal differences in the recruitment of distinct ATM subtypes. The obesity-induced switch in ATM activation state is coupled to the localized recruitment of an inflammatory ATM subtype to macrophage clusters from the circulation and not to the conversion of resident M2a macrophages to M1 ATMs in situ.
Figures
Similar articles
-
MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity.J Exp Med. 2009 Dec 21;206(13):3143-56. doi: 10.1084/jem.20091333. Epub 2009 Dec 7. J Exp Med. 2009. PMID: 19995956 Free PMC article.
-
Obesity induces a phenotypic switch in adipose tissue macrophage polarization.J Clin Invest. 2007 Jan;117(1):175-84. doi: 10.1172/JCI29881. J Clin Invest. 2007. PMID: 17200717 Free PMC article.
-
Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity.Diabetes. 2007 Jan;56(1):16-23. doi: 10.2337/db06-1076. Diabetes. 2007. PMID: 17192460
-
Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states.Curr Opin Clin Nutr Metab Care. 2011 Jul;14(4):341-6. doi: 10.1097/MCO.0b013e328347970b. Curr Opin Clin Nutr Metab Care. 2011. PMID: 21587064 Free PMC article. Review.
-
Adipose tissue macrophages: Unique polarization and bioenergetics in obesity.Immunol Rev. 2020 May;295(1):101-113. doi: 10.1111/imr.12853. Epub 2020 Apr 1. Immunol Rev. 2020. PMID: 32237081 Free PMC article. Review.
Cited by
-
Innate immune activation in obesity.Mol Aspects Med. 2013 Feb;34(1):12-29. doi: 10.1016/j.mam.2012.10.002. Epub 2012 Oct 13. Mol Aspects Med. 2013. PMID: 23068074 Free PMC article. Review.
-
Identification of pigment epithelium-derived factor as an adipocyte-derived inflammatory factor.Mol Med. 2012 Oct 24;18(1):1161-8. doi: 10.2119/molmed.2012.00156. Mol Med. 2012. PMID: 22714715 Free PMC article.
-
Macrophages, meta-inflammation, and immuno-metabolism.ScientificWorldJournal. 2011;11:2509-29. doi: 10.1100/2011/397971. Epub 2011 Dec 28. ScientificWorldJournal. 2011. PMID: 22235182 Free PMC article. Review.
-
CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status.Diabetes. 2012 Jul;61(7):1680-90. doi: 10.2337/db11-1506. Epub 2012 Apr 3. Diabetes. 2012. PMID: 22474027 Free PMC article.
-
Cytokine Output of Adipocyte-iNKT Cell Interplay Is Skewed by a Lipid-Rich Microenvironment.Front Endocrinol (Lausanne). 2020 Jul 31;11:479. doi: 10.3389/fendo.2020.00479. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32849273 Free PMC article.
References
-
- Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A: Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 92 :2240 –2247,2007 - PubMed
-
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M: IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11 :191 –198,2005 - PubMed
-
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS: Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389 :610 –614,1997 - PubMed
-
- Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, Schwendener RA, Brekken RA, Kang G, Oike Y, Choi TS, Suda T, Yoo OJ, Koh GY: Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res 100 :e47 –e57,2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

