Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes
- PMID: 18827013
- PMCID: PMC2586290
- DOI: 10.1242/jcs.031484
Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes
Abstract
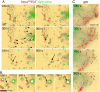
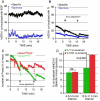
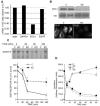
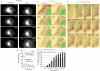
The biological function of receptors is determined by their appropriate trafficking through the endosomal pathway. Following internalization, the transferrin (Tf) receptor quantitatively recycles to the plasma membrane, whereas the epidermal growth factor (EGF) receptor undergoes degradation. To determine how Tf and EGF engage these two different pathways we imaged their binding and early endocytic pathway in live cells using total internal reflection fluorescence microscopy (TIRF-M). We find that EGF and Tf bind to distinct plasma membrane regions and are incorporated into different endocytic vesicles. After internalization, both EGF-enriched and Tf-enriched vesicles interact with endosomes containing early endosome antigen 1 (EEA1). EGF is incorporated and retained in these endosomes, while Tf-containing vesicles rapidly dissociate and move to a juxtanuclear compartment. Endocytic vesicles carrying EGF recruit more Rab5 GTPase than those carrying Tf, which, by strengthening their association with EEA1-enriched endosomes, may provide a mechanism for the observed cargo-specific sorting. These results reveal pre-endocytic sorting of Tf and EGF, a specialized role for EEA1-enriched endosomes in EGF trafficking, and a potential mechanism for cargo-specified sorting of endocytic vesicles by these endosomes.
Figures


Similar articles
-
Visualization of self-delivering hydrophobically modified siRNA cellular internalization.Nucleic Acids Res. 2017 Jan 9;45(1):15-25. doi: 10.1093/nar/gkw1005. Epub 2016 Nov 28. Nucleic Acids Res. 2017. PMID: 27899655 Free PMC article.
-
ESCRT-0 marks an APPL1-independent transit route for EGFR between the cell surface and the EEA1-positive early endosome.J Cell Sci. 2015 Feb 15;128(4):755-67. doi: 10.1242/jcs.161786. Epub 2015 Jan 14. J Cell Sci. 2015. PMID: 25588841 Free PMC article.
-
Dynein is required for receptor sorting and the morphogenesis of early endosomes.Nat Cell Biol. 2007 Jan;9(1):113-20. doi: 10.1038/ncb1525. Epub 2006 Dec 17. Nat Cell Biol. 2007. PMID: 17173037
-
Endocytic recycling in plants: pathways and regulation.J Exp Bot. 2024 Aug 28;75(16):4712-4728. doi: 10.1093/jxb/erae188. J Exp Bot. 2024. PMID: 38655916 Review.
-
Redox Signaling in Endosomes Using the Example of EGF Receptors: A Graphical Review.Antioxidants (Basel). 2024 Oct 9;13(10):1215. doi: 10.3390/antiox13101215. Antioxidants (Basel). 2024. PMID: 39456468 Free PMC article. Review.
Cited by
-
Regulation of the epithelial sodium channel (ENaC) by membrane trafficking.Biochim Biophys Acta. 2010 Dec;1802(12):1166-77. doi: 10.1016/j.bbadis.2010.03.010. Epub 2010 Mar 27. Biochim Biophys Acta. 2010. PMID: 20347969 Free PMC article. Review.
-
Protein interacting with Amyloid Precursor Protein tail-1 (PAT1) is involved in early endocytosis.Cell Mol Life Sci. 2019 Dec;76(24):4995-5009. doi: 10.1007/s00018-019-03157-7. Epub 2019 May 28. Cell Mol Life Sci. 2019. PMID: 31139847 Free PMC article.
-
Transferrin: Endocytosis and Cell Signaling in Parasitic Protozoa.Biomed Res Int. 2015;2015:641392. doi: 10.1155/2015/641392. Epub 2015 May 18. Biomed Res Int. 2015. PMID: 26090431 Free PMC article. Review.
-
Dynamic visualization of membrane-inserted fraction of pHluorin-tagged channels using repetitive acidification technique.BMC Neurosci. 2009 Nov 30;10:141. doi: 10.1186/1471-2202-10-141. BMC Neurosci. 2009. PMID: 19948025 Free PMC article.
-
Clathrin couture: fashioning distinctive membrane coats at the cell surface.PLoS Biol. 2009 Sep;7(9):e1000192. doi: 10.1371/journal.pbio.1000192. Epub 2009 Sep 8. PLoS Biol. 2009. PMID: 19809570 Free PMC article. No abstract available.
References
-
- Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Traffic. 2001;2:764–74. - PubMed
-
- Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 2003;361:1–33. - PubMed
-
- Barbieri MA, Fernandez-Pol S, Hunker C, Horazdovsky BH, Stahl PD. Role of rab5 in EGF receptor-mediated signal transduction. Eur J Cell Biol. 2004;83:305–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

