Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole
- PMID: 18825147
- PMCID: PMC3347761
- DOI: 10.1038/mp.2008.106
Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole
Abstract
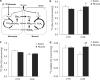
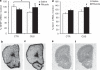
Growing evidence indicates that glia pathology and amino-acid neurotransmitter system abnormalities contribute to the pathophysiology and possibly the pathogenesis of major depressive disorder. This study investigates changes in glial function occurring in the rat prefrontal cortex (PFC) after chronic unpredictable stress (CUS), a rodent model of depression. Furthermore, we analyzed the effects of riluzole, a Food and Drug Administration-approved drug for the treatment of amyotrophic laterosclerosis, known to modulate glutamate release and facilate glutamate uptake, on CUS-induced glial dysfunction and depressive-like behaviors. We provide the first experimental evidence that chronic stress impairs cortical glial function. Animals exposed to CUS and showing behavioral deficits in sucrose preference and active avoidance exhibited significant decreases in 13C-acetate metabolism reflecting glial cell metabolism, and glial fibrillary associated protein (GFAP) mRNA expression in the PFC. The cellular, metabolic and behavioral alterations induced by CUS were reversed and/or blocked by chronic treatment with the glutamate-modulating drug riluzole. The beneficial effects of riluzole on CUS-induced anhedonia and helplessness demonstrate the antidepressant action of riluzole in rodents. Riluzole treatment also reversed CUS-induced reductions in glial metabolism and GFAP mRNA expression. Our results are consistent with recent open-label clinical trials showing the drug's effect in mood and anxiety disorders. This study provides further validation of hypothesis that glial dysfunction and disrupted amino-acid neurotransmission contribute to the pathophysiology of depression and that modulation of glutamate metabolism, uptake and/or release represent viable targets for antidepressant drug development.
Figures
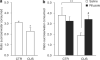

Similar articles
-
Clomipramine treatment reversed the glial pathology in a chronic unpredictable stress-induced rat model of depression.Eur Neuropsychopharmacol. 2009 Nov;19(11):796-805. doi: 10.1016/j.euroneuro.2009.06.010. Epub 2009 Jul 18. Eur Neuropsychopharmacol. 2009. PMID: 19616923
-
Magnolol treatment reversed the glial pathology in an unpredictable chronic mild stress-induced rat model of depression.Eur J Pharmacol. 2013 Jul 5;711(1-3):42-9. doi: 10.1016/j.ejphar.2013.04.008. Epub 2013 Apr 28. Eur J Pharmacol. 2013. PMID: 23632393
-
Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats.Neuropsychopharmacology. 2012 Apr;37(5):1305-20. doi: 10.1038/npp.2011.319. Epub 2011 Dec 21. Neuropsychopharmacology. 2012. PMID: 22189291 Free PMC article.
-
Beyond monoamines: glutamatergic function in mood disorders.CNS Spectr. 2005 Oct;10(10):808-19. doi: 10.1017/s1092852900010403. CNS Spectr. 2005. PMID: 16400244 Review.
-
Gliogenesis and glial pathology in depression.CNS Neurol Disord Drug Targets. 2007 Jun;6(3):219-33. doi: 10.2174/187152707780619326. CNS Neurol Disord Drug Targets. 2007. PMID: 17511618 Free PMC article. Review.
Cited by
-
Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond.Biol Psychiatry. 2013 Jun 15;73(12):1133-41. doi: 10.1016/j.biopsych.2013.03.026. Biol Psychiatry. 2013. PMID: 23726151 Free PMC article. Review.
-
From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders.Biol Psychiatry. 2013 Jun 15;73(12):1172-9. doi: 10.1016/j.biopsych.2013.03.032. Biol Psychiatry. 2013. PMID: 23726152 Free PMC article. Review.
-
Astrocyte-derived ATP modulates depressive-like behaviors.Nat Med. 2013 Jun;19(6):773-7. doi: 10.1038/nm.3162. Epub 2013 May 5. Nat Med. 2013. PMID: 23644515
-
Candidate hippocampal biomarkers of susceptibility and resilience to stress in a rat model of depression.Mol Cell Proteomics. 2012 Jul;11(7):M111.016428. doi: 10.1074/mcp.M111.016428. Epub 2012 Feb 6. Mol Cell Proteomics. 2012. PMID: 22311638 Free PMC article.
-
Regulation of a truncated form of tropomyosin-related kinase B (TrkB) by Hsa-miR-185* in frontal cortex of suicide completers.PLoS One. 2012;7(6):e39301. doi: 10.1371/journal.pone.0039301. Epub 2012 Jun 25. PLoS One. 2012. PMID: 22802923 Free PMC article.
References
-
- Schildkaut JJ. The catacholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. - PubMed
-
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. - PubMed
-
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cerebral Cortex. 2002;12:386–394. - PubMed
-
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

