Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients
- PMID: 18822183
- PMCID: PMC2614515
- DOI: 10.1186/bcr2149
Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients
Abstract
Introduction: The phosphoinositide-3 kinase (PI3K)/Akt pathway, operating downstream of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER)2, is implicated in cell migration and survival. EGFR and HER2 are expressed in circulating tumor cells, but the activation status of downstream signaling molecules has not yet been reported.
Methods: To investigate expression levels of EGFR, HER2, PI3K, and Akt in circulating tumor cells, we used peripheral blood mononuclear cells from 32 cytokeratin-19 mRNA-positive patients with early (n = 16) and metastatic (n = 16) breast cancer.Peripheral blood mononuclear cell cytospins were double stained with cytokeratin antibody along with one of the following: EGFR, phospho-EGFR, HER2, phospho-PI3K, or phospho-Akt antibodies.
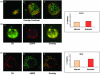
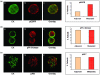
Results: EGFR and HER2 were expressed in circulating tumor cells of 38% and 50% patients with early and 44% and 63% patients with metastatic disease, respectively. Interestingly, phospho-PI3K and phospho-Akt expression levels were similar at 88% (14 out of 16) and 81% (13 out of 16), respectively, in circulating tumor cells of patients with early and metastatic disease. Phospho-EGFR was observed in circulating tumor cells of two (33%) early and six (86%) metastatic EGFR-positive patients. Immunomagnetic separation of peripheral blood mononuclear cells, using EpCAM antibody, and subsequent double-staining experiments of circulating tumor cells showed that EGFR was co-expressed with HER2, phospho-Akt and phospho-PI3K kinases, indicating activation of the corresponding survival signaling pathway.
Conclusions: Our findings demonstrate that circulating tumor cells express receptors and activated signaling kinases of the EGFR/HER2/PI3K/Akt pathway, which could be used as targets for their effective elimination.
Figures

Similar articles
-
Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells.Cancer Res. 2005 Dec 1;65(23):11018-25. doi: 10.1158/0008-5472.CAN-05-0491. Cancer Res. 2005. PMID: 16322251
-
The interplay of HER2/HER3/PI3K and EGFR/HER2/PLC-γ1 signalling in breast cancer cell migration and dissemination.J Pathol. 2012 Jun;227(2):234-44. doi: 10.1002/path.3991. Epub 2012 Mar 21. J Pathol. 2012. PMID: 22262199
-
Phospho-Akt pathway activation and inhibition depends on N-cadherin or phospho-EGFR expression in invasive human bladder cancer cell lines.Urol Oncol. 2010 Mar-Apr;28(2):180-8. doi: 10.1016/j.urolonc.2008.09.041. Epub 2008 Dec 12. Urol Oncol. 2010. PMID: 19070520
-
Natural inhibitors of PI3K/AKT signaling in breast cancer: emphasis on newly-discovered molecular mechanisms of action.Pharmacol Res. 2015 Mar;93:1-10. doi: 10.1016/j.phrs.2014.12.004. Epub 2014 Dec 19. Pharmacol Res. 2015. PMID: 25533812 Review.
-
"The Infinite Maze" of breast cancer, signaling pathways and radioresistance.Breast. 2013 Aug;22(4):411-8. doi: 10.1016/j.breast.2013.04.003. Epub 2013 May 1. Breast. 2013. PMID: 23642528 Review.
Cited by
-
Establishment of a multimarker qPCR panel for the molecular characterization of circulating tumor cells in blood samples of metastatic breast cancer patients during the course of palliative treatment.Oncotarget. 2016 Jul 5;7(27):41677-41690. doi: 10.18632/oncotarget.9528. Oncotarget. 2016. PMID: 27223437 Free PMC article.
-
Tumor-expressed microRNAs associated with venous thromboembolism in colorectal cancer.Res Pract Thromb Haemost. 2022 Jul 1;6(5):e12749. doi: 10.1002/rth2.12749. eCollection 2022 Jul. Res Pract Thromb Haemost. 2022. PMID: 35794963 Free PMC article.
-
Rare cell isolation and profiling on a hybrid magnetic/size-sorting chip.Biomicrofluidics. 2013 Sep 17;7(5):54107. doi: 10.1063/1.4821923. eCollection 2013. Biomicrofluidics. 2013. PMID: 24404070 Free PMC article.
-
pERK/pAkt phenotyping in circulating tumor cells as a biomarker for sorafenib efficacy in patients with advanced hepatocellular carcinoma.Oncotarget. 2016 Jan 19;7(3):2646-59. doi: 10.18632/oncotarget.6104. Oncotarget. 2016. PMID: 26544731 Free PMC article.
-
Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: Mechanisms and clinical applications.Oncotarget. 2017 May 26;8(46):81558-81571. doi: 10.18632/oncotarget.18277. eCollection 2017 Oct 6. Oncotarget. 2017. PMID: 29113414 Free PMC article. Review.
References
-
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. - DOI - PubMed
-
- Kruger W, Krzizanowski C, Holweg M, Stockschlader M, Kroger N, Jung R, Mross K, Jonat W, Zander AR. Reverse transcriptase/polymerase chain reaction detection of cytokeratin-19 mRNA in bone marrow and blood of breast cancer patients. J Cancer Res Clin Oncol. 1996;122:679–686. doi: 10.1007/BF01209032. - DOI - PubMed
-
- Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Kouroussis C, Apostolaki S, Malamos N, Kakolyris S, Kotsakis A, Xenidis N, Reppa D, Georgoulias V. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002;20:3404–3412. doi: 10.1200/JCO.2002.08.135. - DOI - PubMed
-
- Lobodasch K, Frohlich F, Rengsberger M, Schubert R, Dengler R, Pachmann U, Pachmann K. Quantification of circulating tumour cells for the monitoring of adjuvant therapy in breast cancer: an increase in cell number at completion of therapy is a predictor of early relapse. Breast. 2007;16:211–218. doi: 10.1016/j.breast.2006.12.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

