Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules
- PMID: 18819922
- PMCID: PMC2662226
- DOI: 10.1074/jbc.M805729200
Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules
Abstract
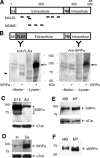
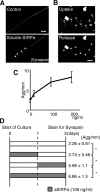
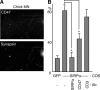
Formation of chemical synapses requires exchange of organizing signals between the synaptic partners. Using synaptic vesicle aggregation in cultured neurons as a marker of presynaptic differentiation, we purified candidate presynaptic organizers from mouse brain. A major bioactive species was the extracellular domain of signal regulatory protein alpha (SIRP-alpha), a transmembrane immunoglobulin superfamily member concentrated at synapses. The extracellular domain of SIRP-alpha is cleaved and shed in a developmentally regulated manner. The presynaptic organizing activity of SIRP-alpha is mediated in part by CD47. SIRP-alpha homologues, SIRP-beta and -gamma also have synaptic vesicle clustering activity. The effects of SIRP-alpha are distinct from those of another presynaptic organizer, FGF22: the two proteins induced vesicle clusters of different sizes, differed in their ability to promote neurite branching, and acted through different receptors and signaling pathways. SIRP family proteins may act together with other organizing molecules to pattern synapses.
Figures


Similar articles
-
SIRP-alpha-CD47 system functions as an intercellular signal in the renal glomerulus.Am J Physiol Renal Physiol. 2010 Sep;299(3):F517-27. doi: 10.1152/ajprenal.00571.2009. Epub 2010 Jun 16. Am J Physiol Renal Physiol. 2010. PMID: 20554646
-
FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain.Cell. 2004 Jul 23;118(2):257-70. doi: 10.1016/j.cell.2004.06.025. Cell. 2004. PMID: 15260994
-
Novel structural determinants on SIRP alpha that mediate binding to CD47.J Immunol. 2007 Dec 1;179(11):7741-50. doi: 10.4049/jimmunol.179.11.7741. J Immunol. 2007. PMID: 18025220
-
Signal regulation by family conspiracy.Cell Mol Life Sci. 2001 Jan;58(1):117-24. doi: 10.1007/PL00000771. Cell Mol Life Sci. 2001. PMID: 11229810 Free PMC article. Review.
-
CD47 in the immune response: role of thrombospondin and SIRP-alpha reverse signaling.Curr Drug Targets. 2008 Oct;9(10):842-50. doi: 10.2174/138945008785909310. Curr Drug Targets. 2008. PMID: 18855618 Review.
Cited by
-
Secreted factors as synaptic organizers.Eur J Neurosci. 2010 Jul;32(2):181-90. doi: 10.1111/j.1460-9568.2010.07338.x. Epub 2010 Jul 14. Eur J Neurosci. 2010. PMID: 20646052 Free PMC article. Review.
-
A New Serum Macrophage Checkpoint Biomarker for Innate Immunotherapy: Soluble Signal-Regulatory Protein Alpha (sSIRPα).Biomolecules. 2022 Jul 4;12(7):937. doi: 10.3390/biom12070937. Biomolecules. 2022. PMID: 35883493 Free PMC article.
-
A Proteome-Wide Effect of PHF8 Knockdown on Cortical Neurons Shows Downregulation of Parkinson's Disease-Associated Protein Alpha-Synuclein and Its Interactors.Biomedicines. 2023 Feb 8;11(2):486. doi: 10.3390/biomedicines11020486. Biomedicines. 2023. PMID: 36831023 Free PMC article.
-
Orchestrating the synaptic network by tyrosine phosphorylation signalling.J Biochem. 2011 Jun;149(6):641-53. doi: 10.1093/jb/mvr047. Epub 2011 Apr 20. J Biochem. 2011. PMID: 21508038 Free PMC article. Review.
-
Tyrosine phosphorylation of the transmembrane protein SIRPα: Sensing synaptic activity and regulating ectodomain cleavage for synapse maturation.J Biol Chem. 2018 Aug 3;293(31):12026-12042. doi: 10.1074/jbc.RA117.001488. Epub 2018 Jun 18. J Biol Chem. 2018. PMID: 29914984 Free PMC article.
References
-
- Sanes, J. R., and Lichtman, J. W. (1999) Annu. Rev. Neurosci. 22 389-442 - PubMed
-
- Waites, C. L., Craig, A. M., and Garner, C. C. (2005) Annu. Rev. Neurosci. 28 251-274 - PubMed
-
- Fox, M. A., and Umemori, H. (2006) J. Neurochem. 97 1215-1231 - PubMed
-
- Scheiffele, P. (2003) Annu. Rev. Neurosci. 26 485-508 - PubMed
-
- Yamagata, M., Sanes, J. R., and Weiner, J. A. (2003) Curr. Opin. Cell Biol. 15 621-632 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

