Activation of the Wnt/beta-catenin signaling reporter in developing mouse olfactory nerve layer marks a specialized subgroup of olfactory ensheathing cells
- PMID: 18816448
- PMCID: PMC2895989
- DOI: 10.1002/dvdy.21712
Activation of the Wnt/beta-catenin signaling reporter in developing mouse olfactory nerve layer marks a specialized subgroup of olfactory ensheathing cells
Abstract
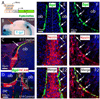
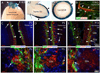
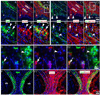
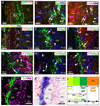
Wnt reporter TOPgal mice carry a beta-galactosidase (betagal) gene under the control of the Wnt/beta-catenin signaling responsive elements. We found that the intensely immunolabeled betagal+ cells were co-immunolabeled with Nestin and formed a tangentially oriented single-cell layer in the "connecting or docking zone" where the olfactory sensory axons attached to the brain surface during mid-gestation. During early postnatal development, betagal+ cells were located in the inner olfactory nerve layer (ONLi) and co-labeled with olfactory ensheathing cell (OEC) markers S100beta and NPY but not with lineage-specific markers for neurons, oligodendrocytes, astrocytes, and microglia, demonstrating that the TOPgal marked a subpopulation of OECs. By confocal microscopy, we found that TOPgal activated processes extended along the developing glomerulus and formed multiple tunnel-like structures that ensheathe and bridge olfactory sensory axonal bundles from ONLi to the glomerulus, which may play a key role in glomerulus formation and convergent sorting of the peripheral olfactory axons.
Copyright (c) 2008 Wiley-Liss, Inc.
Figures
Similar articles
-
A unique cell population in the mouse olfactory bulb displays nuclear beta-catenin signaling during development and olfactory sensory neuron regeneration.Dev Neurobiol. 2008 Jun;68(7):859-69. doi: 10.1002/dneu.20606. Dev Neurobiol. 2008. PMID: 18327767
-
Transcription factor Runx1 inhibits proliferation and promotes developmental maturation in a selected population of inner olfactory nerve layer olfactory ensheathing cells.Gene. 2014 May 1;540(2):191-200. doi: 10.1016/j.gene.2014.02.038. Epub 2014 Feb 26. Gene. 2014. PMID: 24582971
-
Wnt/Frizzled family members mediate olfactory sensory neuron axon extension.J Comp Neurol. 2008 Nov 20;511(3):301-17. doi: 10.1002/cne.21834. J Comp Neurol. 2008. PMID: 18803244 Free PMC article.
-
Axon behavior in the olfactory nerve reflects the involvement of catenin-cadherin mediated adhesion.J Comp Neurol. 2006 Dec 20;499(6):979-89. doi: 10.1002/cne.21147. J Comp Neurol. 2006. PMID: 17072833
-
Sublaminar organization of the mouse olfactory bulb nerve layer.J Comp Neurol. 2002 Apr 22;446(1):68-80. doi: 10.1002/cne.10182. J Comp Neurol. 2002. PMID: 11920721
Cited by
-
Axon guidance events in the wiring of the mammalian olfactory system.Mol Neurobiol. 2009 Feb;39(1):1-9. doi: 10.1007/s12035-008-8047-7. Epub 2008 Dec 2. Mol Neurobiol. 2009. PMID: 19048417 Review.
-
Designing Olfactory Ensheathing Cell Transplantation Therapies: Influence of Cell Microenvironment.Cell Transplant. 2022 Jan-Dec;31:9636897221125685. doi: 10.1177/09636897221125685. Cell Transplant. 2022. PMID: 36124646 Free PMC article. Review.
-
Canonical Wnt signaling activity during synovial joint development.J Mol Histol. 2009 Aug;40(4):311-6. doi: 10.1007/s10735-009-9242-1. Epub 2009 Nov 18. J Mol Histol. 2009. PMID: 19921490
-
Dishevelled proteins are associated with olfactory sensory neuron presynaptic terminals.PLoS One. 2013;8(2):e56561. doi: 10.1371/journal.pone.0056561. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437169 Free PMC article.
-
A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse.BMC Dev Biol. 2010 Dec 21;10:121. doi: 10.1186/1471-213X-10-121. BMC Dev Biol. 2010. PMID: 21176145 Free PMC article.
References
-
- Aoki K, Osumi-Yamashita N, Ninomiya Y, Eto K. Differential expression of NCAM, vimentin and MAP1B during initial pathfinding of olfactory receptor neurons in the mouse embryo. Anat Embryol (Berl) 1995;192:211–220. - PubMed
-
- Au E, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;41:224–236. - PubMed
-
- Au WW, Treloar HB, Greer CA. Sublaminar organization of the mouse olfactory bulb nerve layer. J Comp Neurol. 2002;446:68–80. - PubMed
-
- Bailey MS, Puche AC, Shipley MT. Development of the olfactory bulb: evidence for glia-neuron interactions in glomerular formation. J Comp Neurol. 1999;415:423–448. - PubMed
-
- Balmer CW, LaMantia AS. Noses and neurons: induction, morphogenesis, and neuronal differentiation in the peripheral olfactory pathway. Dev Dyn. 2005;234:464–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

