Kaposi's sarcoma-associated herpesvirus disrupts adherens junctions and increases endothelial permeability by inducing degradation of VE-cadherin
- PMID: 18815301
- PMCID: PMC2583667
- DOI: 10.1128/JVI.01042-08
Kaposi's sarcoma-associated herpesvirus disrupts adherens junctions and increases endothelial permeability by inducing degradation of VE-cadherin
Abstract
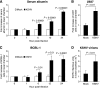
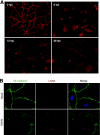
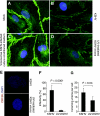
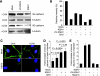
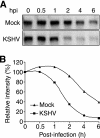
Kaposi's sarcoma (KS) is a vascular tumor of proliferative endothelial cells caused by KS-associated herpesvirus (KSHV) infection. Aberrant vascular permeability is a hallmark of KS manifested as multifocal edematous skin and visceral lesions with dysregulated angiogenesis and vast inflammatory infiltrations. In this study, we showed that KSHV infection increased the permeability of confluent endothelial monolayers to serum albumin, blood-derived cells, KSHV-infected cells, and KSHV virions. KSHV-induced permeability was associated with the disruption of adherens junctions and the degradation of vascular endothelial cadherin (VE-cadherin) protein. Both the inactivation of KSHV virions by UV irradiation and the blockage of de novo protein synthesis with cycloheximide failed to reverse the KSHV-induced disruption of adherens junctions. However, soluble heparin that blocked KSHV entry into cells completely inhibited KSHV-induced permeability. Furthermore, the KSHV-induced degradation of VE-cadherin was dose dependent on the internalized virus particles. Together, these results indicate that KSHV infection induces vascular permeability by inducing VE-cadherin degradation during virus entry into cells. KSHV-induced aberrant vascular permeability could facilitate virus spread, promote inflammation and angiogenesis, and contribute to the pathogenesis of KSHV-induced malignancies.
Figures

Similar articles
-
Latent KSHV infection increases the vascular permeability of human endothelial cells.Blood. 2011 Nov 10;118(19):5344-54. doi: 10.1182/blood-2011-03-341552. Epub 2011 Aug 31. Blood. 2011. PMID: 21881052 Free PMC article.
-
Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells.J Virol. 2010 Jul;84(14):7405-11. doi: 10.1128/JVI.00576-10. Epub 2010 May 12. J Virol. 2010. PMID: 20463083 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus upregulates angiogenin during infection of human dermal microvascular endothelial cells, which induces 45S rRNA synthesis, antiapoptosis, cell proliferation, migration, and angiogenesis.J Virol. 2009 Apr;83(7):3342-64. doi: 10.1128/JVI.02052-08. Epub 2009 Jan 21. J Virol. 2009. PMID: 19158252 Free PMC article.
-
Manipulation of endothelial cells by KSHV: implications for angiogenesis and aberrant vascular differentiation.Semin Cancer Biol. 2014 Jun;26:69-77. doi: 10.1016/j.semcancer.2014.01.008. Epub 2014 Jan 28. Semin Cancer Biol. 2014. PMID: 24486643 Review.
-
VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity.Dev Cell. 2013 Sep 16;26(5):441-54. doi: 10.1016/j.devcel.2013.08.020. Dev Cell. 2013. PMID: 24044891 Review.
Cited by
-
Latent KSHV infection of endothelial cells induces integrin beta3 to activate angiogenic phenotypes.PLoS Pathog. 2011 Dec;7(12):e1002424. doi: 10.1371/journal.ppat.1002424. Epub 2011 Dec 8. PLoS Pathog. 2011. PMID: 22174684 Free PMC article.
-
KSHV Induction of Angiogenic and Lymphangiogenic Phenotypes.Front Microbiol. 2012 Mar 30;3:102. doi: 10.3389/fmicb.2012.00102. eCollection 2012. Front Microbiol. 2012. PMID: 22479258 Free PMC article.
-
Modulation of Angiogenic Processes by the Human Gammaherpesviruses, Epstein-Barr Virus and Kaposi's Sarcoma-Associated Herpesvirus.Front Microbiol. 2019 Jul 12;10:1544. doi: 10.3389/fmicb.2019.01544. eCollection 2019. Front Microbiol. 2019. PMID: 31354653 Free PMC article. Review.
-
Exploitation of Cellular Cytoskeletons and Signaling Pathways for Cell Entry by Kaposi's Sarcoma-Associated Herpesvirus and the Closely Related Rhesus Rhadinovirus.Pathogens. 2012 Dec;1(2):102-27. doi: 10.3390/pathogens1020102. Epub 2012 Oct 22. Pathogens. 2012. PMID: 23420076 Free PMC article.
-
Latent KSHV infection increases the vascular permeability of human endothelial cells.Blood. 2011 Nov 10;118(19):5344-54. doi: 10.1182/blood-2011-03-341552. Epub 2011 Aug 31. Blood. 2011. PMID: 21881052 Free PMC article.
References
-
- Afonso, P. V., S. Ozden, M. C. Prevost, C. Schmitt, D. Seilhean, B. Weksler, P. O. Couraud, A. Gessain, I. A. Romero, and P. E. Ceccaldi. 2007. Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization. J. Immunol. 1792576-2583. - PubMed
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284235-249. - PubMed
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108407-419. - PubMed
-
- Avizienyte, E., A. W. Wyke, R. J. Jones, G. W. McLean, M. A. Westhoff, V. G. Brunton, and M. C. Frame. 2002. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signaling. Nat. Cell Biol. 4632-638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

