Gain-of-function mutations in the transcription factor MRR1 are responsible for overexpression of the MDR1 efflux pump in fluconazole-resistant Candida dubliniensis strains
- PMID: 18809934
- PMCID: PMC2592883
- DOI: 10.1128/AAC.00740-08
Gain-of-function mutations in the transcription factor MRR1 are responsible for overexpression of the MDR1 efflux pump in fluconazole-resistant Candida dubliniensis strains
Abstract
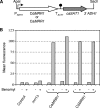
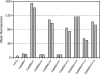
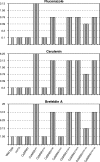
Candida dubliniensis, a yeast that is closely related to Candida albicans, can rapidly develop resistance to the commonly used antifungal agent fluconazole in vitro and in vivo during antimycotic therapy. Fluconazole resistance in C. dubliniensis is usually caused by constitutive overexpression of the MDR1 gene, which encodes a multidrug efflux pump of the major facilitator superfamily. The zinc cluster transcription factor Mrr1p has recently been shown to control MDR1 expression in C. albicans in response to inducing stimuli, and gain-of-function mutations in the MRR1 gene result in constitutive upregulation of the MDR1 efflux pump. We identified a gene with a high degree of similarity to C. albicans MRR1 (CaMRR1) in the C. dubliniensis genome sequence. When C. dubliniensis MRR1 (CdMRR1) was expressed in C. albicans mrr1Delta mutants, it restored benomyl-inducible MDR1 expression, demonstrating that CdMRR1 is the ortholog of CaMRR1. To investigate whether MDR1 overexpression in C. dubliniensis is caused by mutations in MRR1, we sequenced the MRR1 alleles from a fluconazole-resistant, clinical C. dubliniensis isolate and a matched, fluconazole-susceptible isolate from the same patient as well as those from four in vitro-generated, fluconazole-resistant C. dubliniensis strains derived from two different C. dubliniensis isolates. We found that all five resistant strains contained single nucleotide substitutions or small in-frame deletions that resulted in amino acid changes in Mrr1p. Expression of these mutated alleles in C. albicans resulted in the constitutive activation of the MDR1 promoter and multidrug resistance. Therefore, mutations in MRR1 are the major cause of MDR1 upregulation in both C. albicans and C. dubliniensis, demonstrating that the transcription factor Mrr1p plays a central role in the development of drug resistance in these human fungal pathogens.
Figures
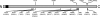
Similar articles
-
The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans.PLoS Pathog. 2007 Nov;3(11):e164. doi: 10.1371/journal.ppat.0030164. PLoS Pathog. 2007. PMID: 17983269 Free PMC article.
-
Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains.Mol Microbiol. 2008 Aug;69(4):827-40. doi: 10.1111/j.1365-2958.2008.06309.x. Epub 2008 May 27. Mol Microbiol. 2008. PMID: 18577180 Free PMC article.
-
Induction of Candida albicans drug resistance genes by hybrid zinc cluster transcription factors.Antimicrob Agents Chemother. 2015 Jan;59(1):558-69. doi: 10.1128/AAC.04448-14. Epub 2014 Nov 10. Antimicrob Agents Chemother. 2015. PMID: 25385116 Free PMC article.
-
The genetic basis of fluconazole resistance development in Candida albicans.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):240-8. doi: 10.1016/s0925-4439(02)00087-x. Biochim Biophys Acta. 2002. PMID: 12084466 Review.
-
Candida dubliniensis, a new fungal pathogen.J Basic Microbiol. 2002;42(3):207-27. doi: 10.1002/1521-4028(200206)42:3<207::AID-JOBM207>3.0.CO;2-C. J Basic Microbiol. 2002. PMID: 12111748 Review.
Cited by
-
Adaptation of Candida albicans to specific host environments by gain-of-function mutations in transcription factors.PLoS Pathog. 2024 Nov 4;20(11):e1012643. doi: 10.1371/journal.ppat.1012643. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39495716 Free PMC article. Review.
-
Mrr1 regulation of methylglyoxal catabolism and methylglyoxal-induced fluconazole resistance in Candida lusitaniae.Mol Microbiol. 2021 Jan;115(1):116-130. doi: 10.1111/mmi.14604. Epub 2020 Dec 14. Mol Microbiol. 2021. PMID: 33319423 Free PMC article.
-
Antifungal resistance and new strategies to control fungal infections.Int J Microbiol. 2012;2012:713687. doi: 10.1155/2012/713687. Epub 2011 Dec 1. Int J Microbiol. 2012. PMID: 22187560 Free PMC article.
-
Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species.Cells. 2023 Nov 19;12(22):2655. doi: 10.3390/cells12222655. Cells. 2023. PMID: 37998390 Free PMC article. Review.
-
The transcription factor Ndt80 does not contribute to Mrr1-, Tac1-, and Upc2-mediated fluconazole resistance in Candida albicans.PLoS One. 2011;6(9):e25623. doi: 10.1371/journal.pone.0025623. Epub 2011 Sep 27. PLoS One. 2011. PMID: 21980509 Free PMC article.
References
-
- Angiolella, L., A. R. Stringaro, F. De Bernardis, B. Posteraro, M. Bonito, L. Toccacieli, A. Torosantucci, M. Colone, M. Sanguinetti, A. Cassone, and A. T. Palamara. 2008. Increase of virulence and its phenotypic traits in drug-resistant strains of Candida albicans. Antimicrob. Agents Chemother. 52:927-936. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

