Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase
- PMID: 18809726
- PMCID: PMC2542475
- DOI: 10.1083/jcb.200801001
Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase
Erratum in
- J Cell Biol. 2011 Dec 12;195(6):1061. Lobinger, Braden T [corrected to Lobingier, Braden T]
Abstract
Rab guanosine triphosphatases (GTPases) are pivotal regulators of membrane identity and dynamics, but the in vivo pathways that control Rab signaling are poorly defined. Here, we show that the GTPase-activating protein Gyp7 inactivates the yeast vacuole Rab Ypt7 in vivo. To efficiently terminate Ypt7 signaling, Gyp7 requires downstream assistance from an inhibitory casein kinase I, Yck3. Yck3 mediates phosphorylation of at least two Ypt7 signaling targets: a tether, the Vps-C/homotypic fusion and vacuole protein sorting (HOPS) subunit Vps41, and a SNARE, Vam3. Phosphorylation of both substrates is opposed by Ypt7-guanosine triphosphate (GTP). We further demonstrate that Ypt7 binds not one but two Vps-C/HOPS subunits: Vps39, a putative Ypt7 nucleotide exchange factor, and Vps41. Gyp7-stimulated GTP hydrolysis on Ypt7 therefore appears to trigger both passive termination of Ypt7 signaling and active kinase-mediated inhibition of Ypt7's downstream targets. We propose that signal propagation through the Ypt7 pathway is controlled by integrated feedback and feed-forward loops. In this model, Yck3 enforces a requirement for the activated Rab in docking and fusion.
Figures


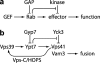
Similar articles
-
The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex.J Cell Biol. 2005 Jan 31;168(3):401-14. doi: 10.1083/jcb.200407141. J Cell Biol. 2005. PMID: 15684030 Free PMC article.
-
New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion.J Cell Biol. 2000 Oct 30;151(3):551-62. doi: 10.1083/jcb.151.3.551. J Cell Biol. 2000. PMID: 11062257 Free PMC article.
-
The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7.Curr Biol. 2010 Sep 28;20(18):1654-9. doi: 10.1016/j.cub.2010.08.002. Curr Biol. 2010. PMID: 20797862
-
Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles.Annu Rev Cell Dev Biol. 2010;26:115-36. doi: 10.1146/annurev-cellbio-100109-104131. Annu Rev Cell Dev Biol. 2010. PMID: 20521906 Review.
-
Membrane dynamics and fusion at late endosomes and vacuoles--Rab regulation, multisubunit tethering complexes and SNAREs.Eur J Cell Biol. 2011 Sep;90(9):779-85. doi: 10.1016/j.ejcb.2011.04.007. Epub 2011 Jun 16. Eur J Cell Biol. 2011. PMID: 21683469 Review.
Cited by
-
Sec17/Sec18 can support membrane fusion without help from completion of SNARE zippering.Elife. 2021 May 4;10:e67578. doi: 10.7554/eLife.67578. Elife. 2021. PMID: 33944780 Free PMC article.
-
Saccharomyces cerevisiae Env7 is a novel serine/threonine kinase 16-related protein kinase and negatively regulates organelle fusion at the lysosomal vacuole.Mol Cell Biol. 2013 Feb;33(3):526-42. doi: 10.1128/MCB.01303-12. Epub 2012 Nov 19. Mol Cell Biol. 2013. PMID: 23166297 Free PMC article.
-
Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization.Nat Genet. 2010 Apr;42(4):303-12. doi: 10.1038/ng.538. Epub 2010 Feb 28. Nat Genet. 2010. PMID: 20190753 Free PMC article.
-
SH3TC2/KIAA1985 protein is required for proper myelination and the integrity of the node of Ranvier in the peripheral nervous system.Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17528-33. doi: 10.1073/pnas.0905523106. Epub 2009 Sep 29. Proc Natl Acad Sci U S A. 2009. PMID: 19805030 Free PMC article.
-
Rab proteins and the secretory pathway: the case of rab18 in neuroendocrine cells.Front Endocrinol (Lausanne). 2011 Jan 17;2:1. doi: 10.3389/fendo.2011.00001. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22649356 Free PMC article.
References
-
- Ali, R., C.L. Brett, S. Mukherjee, and R. Rao. 2004. Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J. Biol. Chem. 279:4498–4506. - PubMed
-
- Bone, N., J.B. Millar, T. Toda, and J. Armstrong. 1998. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr. Biol. 8:135–144. - PubMed
-
- Bos, J.L., H. Rehmann, and A. Wittinghofer. 2007. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 129:865–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM077349-04/GM/NIGMS NIH HHS/United States
- R01 GM077349-03/GM/NIGMS NIH HHS/United States
- P41 RR011823/RR/NCRR NIH HHS/United States
- R01 GM077349-01/GM/NIGMS NIH HHS/United States
- P41 RR11823/RR/NCRR NIH HHS/United States
- GM077349/GM/NIGMS NIH HHS/United States
- P41 RR011823-110095/RR/NCRR NIH HHS/United States
- R01 GM077349-02/GM/NIGMS NIH HHS/United States
- Howard Hughes Medical Institute/United States
- P41 RR011823-148061/RR/NCRR NIH HHS/United States
- R01 GM077349/GM/NIGMS NIH HHS/United States
- P41 RR011823-100095/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

