Innate immunity and intestinal microbiota in the development of Type 1 diabetes
- PMID: 18806780
- PMCID: PMC2574766
- DOI: 10.1038/nature07336
Innate immunity and intestinal microbiota in the development of Type 1 diabetes
Abstract
Type 1 diabetes (T1D) is a debilitating autoimmune disease that results from T-cell-mediated destruction of insulin-producing beta-cells. Its incidence has increased during the past several decades in developed countries, suggesting that changes in the environment (including the human microbial environment) may influence disease pathogenesis. The incidence of spontaneous T1D in non-obese diabetic (NOD) mice can be affected by the microbial environment in the animal housing facility or by exposure to microbial stimuli, such as injection with mycobacteria or various microbial products. Here we show that specific pathogen-free NOD mice lacking MyD88 protein (an adaptor for multiple innate immune receptors that recognize microbial stimuli) do not develop T1D. The effect is dependent on commensal microbes because germ-free MyD88-negative NOD mice develop robust diabetes, whereas colonization of these germ-free MyD88-negative NOD mice with a defined microbial consortium (representing bacterial phyla normally present in human gut) attenuates T1D. We also find that MyD88 deficiency changes the composition of the distal gut microbiota, and that exposure to the microbiota of specific pathogen-free MyD88-negative NOD donors attenuates T1D in germ-free NOD recipients. Together, these findings indicate that interaction of the intestinal microbes with the innate immune system is a critical epigenetic factor modifying T1D predisposition.
Figures
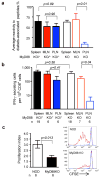
The overall reactivity of T cells from secondary lymphoid organs of MyD88-sufficient (solid bars) and MyD88-negative (open bars) NOD mice was calculated as a mean of the percentages of mice in the given group reacting to individual diabetes-associated peptides (see Supplemental Fig. 2c).
The frequency of CD8+ T cells producing IFN-γ in response to peptide recognized by diabetogenic clone 8.3 was determined in the spleens, MLN and PLN of MyD88-sufficient (solid bars) and MyD88-negative (open bars) NOD mice. Error bars – s.e.m.
Proliferation of CFSE-labeled BDC2.5 CD4+ T cells, depleted of CD25+ T cells, in the PLNs of SPF NOD and NOD.MyD88KO mice assayed on day 3 after i.v. injection. Representative CFSE dilution profiles are shown for BDC2.5+-gated cells in PLN (red) and control skin-draining lymph nodes (blue).
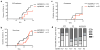
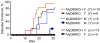
Diabetes incidence in SPF NOD.MyD88KO (J) females and control heterozygous NOD.MyD88KO/+ littermates treated with a broad-spectrum antibiotic from birth.
Diabetes incidence in GF NOD.MyD88KO and control MyD88KO/+ mice. Incidence is shown for male mice; 100% of female NOD.MyD88KO and NOD.MyD88KO/+ female GF mice became diabetic (Supplemental Fig.1).
Diabetes incidence in gnotobiotic male NOD.MyD88KO and control NOD.MyD88KO/+ mice colonized with a consortium of 6 bacterial strains (ASF 361,519,356,492,502, and 500; see Supplemental results for descriptions). The incidence of diabetes in gnotobiotic NOD.MyD88KO mice was significantly different from the incidence in GF NOD.MyD88KO animals (p<0.001), and in gnotobiotic NOD.MyD88KO/+ mice (p<0.05) (Kaplan Meier test).
Histological scores of islet destruction in SPF, GF and ASF-colonized NOD.MyD88KO/+ and NOD.MyD88KO mice. Mice in all groups were males, except for the SPF NOD.MyD88KO group, which included both genders.
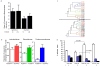
Ratio of Firmicutes to Bacteroidetes in the cecal microbiota of NOD.MyD88KO/+ and NOD.MyD88KO mice who were or were not treated with Sulfatrim. Mean values per mouse ± s.e.m. Untreated NOD.MyD88KO mice had, on average, a significantly lower F/B ratio than all other mice combined (one-tailed t-test, t=-2.31, p=0.013). When comparing post-hoc the effect of sulfatrim in MyD88KO mice only, no significant difference in F/B ratios was observed (t= 0.85, p=0.20).
Abundance of members of three different bacterial families in the cecal contents of NOD.MyD88KO/+ and NOD.MyD88KO mice (not treated with Sulfatrim). Mean values ± s.e.m. Each of the three families is enriched in NOD.MyD88KO mice (Lactobacilliaceae t=-1.54, p=0.07, Porphyromonadaceae t=2.1, p=0.03, Rikenellaceae t=2.74, p=0.007; one-tailed t-test).
Clustering of mouse cecal bacterial communities using the unweighted UniFrac metric n=7,223 sequences. Diversity, not abundance of bacteria was taken into consideration. Top panel: Sulfatrim-treated litters. Bottom panel: Untreated litters. Line colors indicate families. Each label is a mouse: red labels are NOD.MyD88KO/+ (H); black labels are NOD.Myd88KO (K). The number is the common mother, S and N designate exposure to Sulfatrim or no Sulfatrim, respectively.
Histological examination of the pancreata of 8 wk old males from SPF NOD, GF NOD, as well as GF NOD and GF NOD.MyD88KO exposed from birth to microbiota of an SPF NOD.MyD88KO female. The percentages (mean ±s.e.m.) of affected islets with no infiltration (open bars), periinsulitis (blue bars) and true infiltration (combined grades II and III) are shown. p values were obtained using unpaired Student's t test. n, number of animals per group.
Comment in
-
Intestinal microbiota gives a nod to the hygiene hypothesis in type 1 diabetes.Gastroenterology. 2009 Jul;137(1):381-3. doi: 10.1053/j.gastro.2009.05.026. Epub 2009 May 29. Gastroenterology. 2009. PMID: 19482099 No abstract available.
Similar articles
-
Microbiota regulates type 1 diabetes through Toll-like receptors.Proc Natl Acad Sci U S A. 2015 Aug 11;112(32):9973-7. doi: 10.1073/pnas.1508740112. Epub 2015 Jul 27. Proc Natl Acad Sci U S A. 2015. PMID: 26216961 Free PMC article.
-
Differential role of MyD88 and TRIF signaling in myeloid cells in the pathogenesis of autoimmune diabetes.PLoS One. 2018 Mar 9;13(3):e0194048. doi: 10.1371/journal.pone.0194048. eCollection 2018. PLoS One. 2018. PMID: 29522531 Free PMC article.
-
Cutting Edge: Commensal Microbiota Has Disparate Effects on Manifestations of Polyglandular Autoimmune Inflammation.J Immunol. 2016 Aug 1;197(3):701-5. doi: 10.4049/jimmunol.1502465. Epub 2016 Jun 20. J Immunol. 2016. PMID: 27324130 Free PMC article.
-
The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes.J Autoimmun. 2016 Jan;66:76-88. doi: 10.1016/j.jaut.2015.08.019. Epub 2015 Sep 26. J Autoimmun. 2016. PMID: 26403950 Free PMC article. Review.
-
Toll-like receptors and type 1 diabetes.Adv Exp Med Biol. 2010;654:585-610. doi: 10.1007/978-90-481-3271-3_25. Adv Exp Med Biol. 2010. PMID: 20217515 Review.
Cited by
-
Lymphotoxin signalling in immune homeostasis and the control of microorganisms.Nat Rev Immunol. 2013 Apr;13(4):270-9. doi: 10.1038/nri3406. Nat Rev Immunol. 2013. PMID: 23524463 Free PMC article. Review.
-
Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans.Cell. 2016 May 5;165(4):842-53. doi: 10.1016/j.cell.2016.04.007. Epub 2016 Apr 28. Cell. 2016. PMID: 27133167 Free PMC article.
-
Impact of the gut microbiota on rodent models of human disease.World J Gastroenterol. 2014 Dec 21;20(47):17727-36. doi: 10.3748/wjg.v20.i47.17727. World J Gastroenterol. 2014. PMID: 25548471 Free PMC article. Review.
-
MyD88-deficient Hydra reveal an ancient function of TLR signaling in sensing bacterial colonizers.Proc Natl Acad Sci U S A. 2012 Nov 20;109(47):19374-9. doi: 10.1073/pnas.1213110109. Epub 2012 Oct 29. Proc Natl Acad Sci U S A. 2012. PMID: 23112184 Free PMC article.
-
Regulation of Autoimmunity by the Microbiome.DNA Cell Biol. 2016 Sep;35(9):455-8. doi: 10.1089/dna.2016.3432. Epub 2016 Jul 27. DNA Cell Biol. 2016. PMID: 27463238 Free PMC article.
References
-
- Karvonen M, Tuomilehto J, Libman I, LaPorte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia. 1993;36:883–92. - PubMed
-
- Patterson CC, Dahlquist G, Soltesz G, Green A. Is childhood-onset type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia. 2001;44(3):B9–16. - PubMed
-
- Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world--recent facts and figures. Immunol Today. 1993;14:193–6. - PubMed
-
- McInerney MF, Pek SB, Thomas DW. Prevention of insulitis and diabetes onset by treatment with complete Freund's adjuvant in NOD mice. Diabetes. 1991;40:715–25. - PubMed
-
- Sadelain MW, Qin HY, Lauzon J, Singh B. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes. 1990;39:583–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- DK063452/DK/NIDDK NIH HHS/United States
- R21 DK063452-02/DK/NIDDK NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- P30 DK042086-16/DK/NIDDK NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- R01 DK030292/DK/NIDDK NIH HHS/United States
- R01 DK070977-04/DK/NIDDK NIH HHS/United States
- R01 DK070977/DK/NIDDK NIH HHS/United States
- R37 AI046643/AI/NIAID NIH HHS/United States
- P30 DK056341-08/DK/NIDDK NIH HHS/United States
- P30 DK063720-01/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P30 DK056341-07/DK/NIDDK NIH HHS/United States
- P30 DK63720/DK/NIDDK NIH HHS/United States
- DK45735/DK/NIDDK NIH HHS/United States
- DK42086/DK/NIDDK NIH HHS/United States
- DK30292/DK/NIDDK NIH HHS/United States
- R01 DK030292-24/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- DK70977/DK/NIDDK NIH HHS/United States
- R37 AI046643-10/AI/NIAID NIH HHS/United States
- P30 DK045735-10/DK/NIDDK NIH HHS/United States
- R37 AI46643/AI/NIAID NIH HHS/United States
- R21 DK063452/DK/NIDDK NIH HHS/United States
- R37 DK030292/DK/NIDDK NIH HHS/United States
- P30 DK045735-119006/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

